Information to Users
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Excesss Karaoke Master by Artist
XS Master by ARTIST Artist Song Title Artist Song Title (hed) Planet Earth Bartender TOOTIMETOOTIMETOOTIM ? & The Mysterians 96 Tears E 10 Years Beautiful UGH! Wasteland 1999 Man United Squad Lift It High (All About 10,000 Maniacs Candy Everybody Wants Belief) More Than This 2 Chainz Bigger Than You (feat. Drake & Quavo) [clean] Trouble Me I'm Different 100 Proof Aged In Soul Somebody's Been Sleeping I'm Different (explicit) 10cc Donna 2 Chainz & Chris Brown Countdown Dreadlock Holiday 2 Chainz & Kendrick Fuckin' Problems I'm Mandy Fly Me Lamar I'm Not In Love 2 Chainz & Pharrell Feds Watching (explicit) Rubber Bullets 2 Chainz feat Drake No Lie (explicit) Things We Do For Love, 2 Chainz feat Kanye West Birthday Song (explicit) The 2 Evisa Oh La La La Wall Street Shuffle 2 Live Crew Do Wah Diddy Diddy 112 Dance With Me Me So Horny It's Over Now We Want Some Pussy Peaches & Cream 2 Pac California Love U Already Know Changes 112 feat Mase Puff Daddy Only You & Notorious B.I.G. Dear Mama 12 Gauge Dunkie Butt I Get Around 12 Stones We Are One Thugz Mansion 1910 Fruitgum Co. Simon Says Until The End Of Time 1975, The Chocolate 2 Pistols & Ray J You Know Me City, The 2 Pistols & T-Pain & Tay She Got It Dizm Girls (clean) 2 Unlimited No Limits If You're Too Shy (Let Me Know) 20 Fingers Short Dick Man If You're Too Shy (Let Me 21 Savage & Offset &Metro Ghostface Killers Know) Boomin & Travis Scott It's Not Living (If It's Not 21st Century Girls 21st Century Girls With You 2am Club Too Fucked Up To Call It's Not Living (If It's Not 2AM Club Not -

MUSIC NOTES: Exploring Music Listening Data As a Visual Representation of Self
MUSIC NOTES: Exploring Music Listening Data as a Visual Representation of Self Chad Philip Hall A thesis submitted in partial fulfillment of the requirements for the degree of: Master of Design University of Washington 2016 Committee: Kristine Matthews Karen Cheng Linda Norlen Program Authorized to Offer Degree: Art ©Copyright 2016 Chad Philip Hall University of Washington Abstract MUSIC NOTES: Exploring Music Listening Data as a Visual Representation of Self Chad Philip Hall Co-Chairs of the Supervisory Committee: Kristine Matthews, Associate Professor + Chair Division of Design, Visual Communication Design School of Art + Art History + Design Karen Cheng, Professor Division of Design, Visual Communication Design School of Art + Art History + Design Shelves of vinyl records and cassette tapes spark thoughts and mem ories at a quick glance. In the shift to digital formats, we lost physical artifacts but gained data as a rich, but often hidden artifact of our music listening. This project tracked and visualized the music listening habits of eight people over 30 days to explore how this data can serve as a visual representation of self and present new opportunities for reflection. 1 exploring music listening data as MUSIC NOTES a visual representation of self CHAD PHILIP HALL 2 A THESIS SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF: master of design university of washington 2016 COMMITTEE: kristine matthews karen cheng linda norlen PROGRAM AUTHORIZED TO OFFER DEGREE: school of art + art history + design, division -

Eyez on Me 2PAC – Changes 2PAC - Dear Mama 2PAC - I Ain't Mad at Cha 2PAC Feat
2 UNLIMITED- No Limit 2PAC - All Eyez On Me 2PAC – Changes 2PAC - Dear Mama 2PAC - I Ain't Mad At Cha 2PAC Feat. Dr DRE & ROGER TROUTMAN - California Love 311 - Amber 311 - Beautiful Disaster 311 - Down 3 DOORS DOWN - Away From The Sun 3 DOORS DOWN – Be Like That 3 DOORS DOWN - Behind Those Eyes 3 DOORS DOWN - Dangerous Game 3 DOORS DOWN – Duck An Run 3 DOORS DOWN – Here By Me 3 DOORS DOWN - Here Without You 3 DOORS DOWN - Kryptonite 3 DOORS DOWN - Landing In London 3 DOORS DOWN – Let Me Go 3 DOORS DOWN - Live For Today 3 DOORS DOWN – Loser 3 DOORS DOWN – So I Need You 3 DOORS DOWN – The Better Life 3 DOORS DOWN – The Road I'm On 3 DOORS DOWN - When I'm Gone 4 NON BLONDES - Spaceman 4 NON BLONDES - What's Up 4 NON BLONDES - What's Up ( Acoustative Version ) 4 THE CAUSE - Ain't No Sunshine 4 THE CAUSE - Stand By Me 5 SECONDS OF SUMMER - Amnesia 5 SECONDS OF SUMMER - Don't Stop 5 SECONDS OF SUMMER – Good Girls 5 SECONDS OF SUMMER - Jet Black Heart 5 SECONDS OF SUMMER – Lie To Me 5 SECONDS OF SUMMER - She Looks So Perfect 5 SECONDS OF SUMMER - Teeth 5 SECONDS OF SUMMER - What I Like About You 5 SECONDS OF SUMMER - Youngblood 10CC - Donna 10CC - Dreadlock Holiday 10CC - I'm Mandy ( Fly Me ) 10CC - I'm Mandy Fly Me 10CC - I'm Not In Love 10CC - Life Is A Minestrone 10CC - Rubber Bullets 10CC - The Things We Do For Love 10CC - The Wall Street Shuffle 30 SECONDS TO MARS - Closer To The Edge 30 SECONDS TO MARS - From Yesterday 30 SECONDS TO MARS - Kings and Queens 30 SECONDS TO MARS - Teeth 30 SECONDS TO MARS - The Kill (Bury Me) 30 SECONDS TO MARS - Up In The Air 30 SECONDS TO MARS - Walk On Water 50 CENT - Candy Shop 50 CENT - Disco Inferno 50 CENT - In Da Club 50 CENT - Just A Lil' Bit 50 CENT - Wanksta 50 CENT Feat. -

N° Artiste Titre Formatdate Modiftaille 14152 Paul Revere & the Raiders Hungry Kar 2001 42 277 14153 Paul Severs Ik Ben
N° Artiste Titre FormatDate modifTaille 14152 Paul Revere & The Raiders Hungry kar 2001 42 277 14153 Paul Severs Ik Ben Verliefd Op Jou kar 2004 48 860 14154 Paul Simon A Hazy Shade Of Winter kar 1995 18 008 14155 Me And Julio Down By The Schoolyard kar 2001 41 290 14156 You Can Call Me Al kar 1997 83 142 14157 You Can Call Me Al mid 2011 24 148 14158 Paul Stookey I Dig Rock And Roll Music kar 2001 33 078 14159 The Wedding Song kar 2001 24 169 14160 Paul Weller Remember How We Started kar 2000 33 912 14161 Paul Young Come Back And Stay kar 2001 51 343 14162 Every Time You Go Away mid 2011 48 081 14163 Everytime You Go Away (2) kar 1998 50 169 14164 Everytime You Go Away kar 1996 41 586 14165 Hope In A Hopeless World kar 1998 60 548 14166 Love Is In The Air kar 1996 49 410 14167 What Becomes Of The Broken Hearted kar 2001 37 672 14168 Wherever I Lay My Hat (That's My Home) kar 1999 40 481 14169 Paula Abdul Blowing Kisses In The Wind kar 2011 46 676 14170 Blowing Kisses In The Wind mid 2011 42 329 14171 Forever Your Girl mid 2011 30 756 14172 Opposites Attract mid 2011 64 682 14173 Rush Rush mid 2011 26 932 14174 Straight Up kar 1994 21 499 14175 Straight Up mid 2011 17 641 14176 Vibeology mid 2011 86 966 14177 Paula Cole Where Have All The Cowboys Gone kar 1998 50 961 14178 Pavarotti Carreras Domingo You'll Never Walk Alone kar 2000 18 439 14179 PD3 Does Anybody Really Know What Time It Is kar 1998 45 496 14180 Peaches Presidents Of The USA kar 2001 33 268 14181 Pearl Jam Alive mid 2007 71 994 14182 Animal mid 2007 17 607 14183 Better -

The Time Traveler's Wife
When Henry meets Clare, he is twenty-eight and she is twenty. He is a hip librarian; she is a beautiful art student. Henry has never met Clare before; Clare has known Henry since she was six... “A powerfully original love story. BOTTOM LINE: Amazing trip.” —PEOPLE “To those who say there are no new love stories, I heartily recommend The Time Traveler’s Wife, an enchanting novel, which is beautifully crafted and as dazzlingly imaginative as it is dizzyingly romantic.” —SCOTT TUROW AUDREY NIFFENEGGER’S innovative debut, The Time Traveler’s Wife, is the story, of Clare, a beautiful art student, and Henry, an adventuresome librarian, who have known each other since Clare was six and Henry was thirty-six, and were married when Clare was twenty-three and Henry thirty-one. Impossible but true, because Henry is one of the first people diagnosed with Chrono-Displacement Disorder: periodically his genetic clock resets and he finds himself misplaced in time, pulled to moments of emotional gravity from his life, past and future. His disappearances are spontaneous, his experiences unpredictable, alternately harrowing and amusing. The Time Traveler’s Wife depicts the effects of time travel on Henry and Clare’s marriage and their passionate love for each other, as the story unfolds from both points of view. Clare and Henry attempt to live normal lives, pursuing familiar goals— steady jobs, good friends, children of their own. All of this is threatened by something they can neither prevent nor control, making their story intensely moving and entirely unforgettable. THE TIME TRAVELER’S WIFE a novel by Audrey Niffenegger Clock time is our bank manager, tax collector, police inspector; this inner time is our wife. -
Fellowship of Sergeons
Fellowship Surgeons A History of the American College of Surgeons BY LOYAL DAVIS E GE ---- 0/, ' j Tz_ OMNIBVS PER ARTEM FIDEMQVE PRO DESSE American College of Surgeons Fellowship of Surgeons Fellowship of Surgeons A History of the American College of Surgeons BY LOYAL DAVIS, M.D., F.A.C.S. American College of Surgeons AMERICAN COLLEGE OF SURGEONS 55 East Erie Street, Chicago, Illinois 60611-2797 ©1960, by Charles C. Thomas • Publisher ©1973, by American College of Surgeons. ©1981, by American College of Surgeons. ©1984, by American College of Surgeons. ©1988, by American College of Surgeons. ©1993, by American College of Surgeons. All rights reserved. Library of Congress Catalog Number: 59-15598 Printed in the United States of America FOREWORD The most conscientious historian must deal with legends, and legends grow rapidly. Even the passage of a day begins to turn facts into fanciful and entertaining stories. Interestingly told, these tales combine truth and ridiculousness in such delightful and charming proportions that they are bound to last for a long time. The story of the American College of Surgeons is that of the development and progress of surgery in America. No other medical organization, voluntarily entered into by its Fellows, has exerted such a profound influence upon the discipline and art of surgery in the United States. This book is concerned with the many men, each with his in- cisive personality, who were dedicated to the purpose of ele- vating the level of the surgical treatment of patients by raising the standards of hospital facilities and continuing the education of the surgeon. -

Investigation Into the Unlawful Death of Mr. Jamal Khashoggi*
A/HRC/41/CRP.1 19 June 2019 English only Human Rights Council Forty-first session 24 June - 12 July 2019 Agenda item 3 Promotion and protection of all human rights, civil, Political, economic, social and cultural rights, including the right to development Annex to the Report of the Special Rapporteur on extrajudicial, summary or arbitrary executions: Investigation into the unlawful death of Mr. Jamal Khashoggi* * Reproduced as received. GE.19-10061(E) A/HRC/41/CRP.1 Contents Page Executive summary ....................................................................................................................... 4 Introduction ................................................................................................................................... 8 PART I. THE TIMELINE OF THE EXECUTION OF JAMAL KHASHOGGI ................. 14 Allegations of Surveillance of Jamal Khashoggi and Others by Saudi Arabia ............................. 14 Before the murder .......................................................................................................................... 14 Planning and preparation ............................................................................................................... 15 The disappearance and murder of Jamal Khashoggi ..................................................................... 19 The Turkish authorities learn of Mr. Khashoggi’s disappearance. ................................................ 21 Saudi 15 member-team leaves Turkey ......................................................................................... -

CPE Manual of Administration (MOA) Describes Specific Sections and Skills to Be Assessed and Serves As the Guide for Administering the CPE to All Candidates
MANUAL OF ADMINISTRATION for the CLINICAL PROFICIENCY EXAMINATION 2021 Edition (version January 1, 2021) For use ONLY in CPE administrations in calendar year 2021 AVMA Educational Commission for Foreign Veterinary Graduates Canadian National Examining Board MANUAL OF ADMINISTRATION 2021 Table of Contents Table of Contents for the Manual of Administration GENERAL INFORMATION ...................................................................................................................... 1 Statement of Intent ................................................................................................................................... 1 Animal Requirements ............................................................................................................................... 1 Personnel Requirements ........................................................................................................................... 2 Facilities and Equipment.......................................................................................................................... 3 Taking Breaks .......................................................................................................................................... 3 Other Policy Information ......................................................................................................................... 3 General Discussion about the CPE .......................................................................................................... 3 Disabled or Pregnant -

3 Doors Down
3 Doors Down - Here Without You 3 Doors Down - It's Not My Time 3 Doors Down - Kryptonite (2) 3 Doors Down - Kryptonite 3 Doors Down - When I'm Gone 30 Seconds To Mars - The Kill A Day To Remember - 2nd Sucks A Day To Remember - All I Want A Day To Remember - All Signs Point To Lauderdale A Day To Remember - Downfall Of Us All A Day To Remember - Have Faith In Me A Day To Remember - If It Means A Lot To You Ver2 A Day To Remember - If It Means A Lot To You A Day To Remember - Nj Legion Iced Tea A Day To Remember - The Downfall Of Us All Accept - Fast As A Shark ACDC - Shake Your Foundations ACDC - Back In Black ACDC - Big Gun ACDC - Highway To Hell ACDC - Shot Down In Flames ACDC - Stiff Upper Lip ACDC - T. N. T. Ace Frehley - New York Groove Aerosmith - Big Ten Inch Record Aerosmith - Cryin' Aerosmith - Don't Want To Miss A Thing Aerosmith - Dream On Aerosmith - Dude (2) Aerosmith - Dude Aerosmith - Hangman Jury Aerosmith - Hole In My Soul Aerosmith - I Don't Wanna Miss A Thing Aerosmith - I Don't Want To Miss A Thing Aerosmith - Jaded Aerosmith - Janie's Got A Gun Aerosmith - Just Push Play Aerosmith - Living On The Edge Aerosmith - Love In An Eleator Aerosmith - Rag Doll Aerosmith - Remember Aerosmith - Sweet Emotion Aerosmith - Toys In The Attic Aerosmith - What It Takes Aerosmith -Same Old Song And Dance Aerosmith- Back In The Saddle Alcatrazz - God Blessed Alestorm - Shipwrecked Alice Cooper - Is It My Body Alice Cooper - Last Man On Earth Alice Cooper - Lost In America Alice Cooper - No More Mr Nice Guy Alice Cooper - Poison Alice -

W Dzisiejszym Numerze
Gazetka uczniów SLO im. sw. Dominika Savio we Wroclawiu Numer 01/08 Marzec 2008 „Chrystus zmartwychwstał prawdziwie i my jesteśmy tego świadkami! Krzyczymy o tym światu, aby nasza radość dotarła do wielu innych serc, zapalając w nich światło nadziei, która nie zawodzi” (Jan Paweł II) DRODZY UCZNIOWIE I RODZICE, NAUCZYCIELE I I PRACOWNICY , BYLI WYCHOWANKOWIE ORAZ SYMPATYCY LICEUM SALEZJAŃSKIEGO WE WROCŁAWIU! W najcudowniejszym dniu naszej wiary, gdy Jezus z miłości dał nam nadzieję życia wiecznego w szczęściu życzę Wam, aby radość jak i moc Bożej łaski pomagały odważnie i ufnie podążać każdego dnia drogą prawdy do Nieba. z modlitwą ks. Jerzy Babiak dyrektor W dzisiejszym numerze: Życzenia Wielkanocne.................................str. 1 Step Up 2 - recenzja.....................................str. 2 O koniach.....................................................str. 2 Być kobietą..................................................str. 2-3 Życie wg. Mateusza.....................................str. 4 Wielkanoc................... ................................str. 4 Czy na pewno święta?.................................str. 4 Porcupine Tree............................................str. 4-5 Przepis na uśmiech......................................str. 6 Wszyscy pomagają......................................str. 6 Rekolekcje szkolna......................................str. 6-7 Relacja z Hiszpanii......................................str. 8 Nasze sukcesy..............................................str. 8 Numer 01/08 Marzec 2008 1 Taniec -
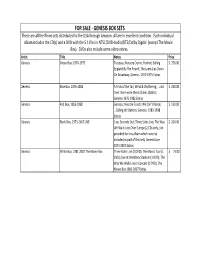
FOR SALE ‐ GENESIS BOX SETS These Are All the Rhino Sets Distributed to the USA Through Amazon
FOR SALE ‐ GENESIS BOX SETS These are all the Rhino sets distributed to the USA through Amazon. All are in excellent condition. Each individual album includes the CD(s) and a DVD with the 5.1 files in NTSC/DVD‐Audio/DTS/Dolby Digital (except The Movie Box). DVDs also include some video extras. Artist Title Notes Price Genesis Green Box, 1970‐1975 Trespass; Nursery Cryme; Foxtrot; Selling $ 250.00 England By The Pound; The Lamb Lies Down On Broadway; Genesis 1970‐1975 Extras Genesis Blue Box, 1976‐1982 A Trick of the Tail; Wind & Wuthering; …and $ 250.00 then there were three; Duke; ababab; Genesis 1976‐1982 Extras Genesis Red Box, 1983‐1998 Genesis; Invisible Touch; We Can’t Dance; $ 150.00 …Calling All Stations; Genesis 1983‐1998 Extras Genesis Black Box, 1973‐2007 LIVE Live; Seconds Out; Three Sides Live; The Way $ 200.00 We Walk; Live Over Europe (2 CDs only, slot provided for this album which was not included as part of this set); Genesis Live 1973‐2007 Extras Genesis White Box, 1981‐2007 The Movie Box Three Sides Live (1 DVD); The Mama Tour (1 $ 75.00 DVD); Live at Wembley Stadium (1 DVD); The Way We Walk Live in Concert (1 DVD); The Movie Box 1981‐2007 Extras FOR SALE ‐ SACD STEREO Artist Title Notes Price SOLD ‐ Boston Boston $ 12.00 Chicago Symphony/Dvorak New World Symphony $ 7.00 Dylan, Bob The Free Wheelin' Bob Dylan $ 12.00 Dylan, Bob Highway 61 revisited $ 10.00 Guaraldi Trio, Vince A Charlie Brown Christmas SACD Only $ 12.00 SOLD ‐ Journey Escape SACD Only $ 12.00 SOLD ‐ The Police Synchronicity SACD Only $ 15.00 SOLD ‐ -

UWM Libraries Digital Collections
Part 2 of the story of one UWM Absolutely no talking Job search a little slow? student's struggle with cancer during these films PAGE 6 PAGE 11 PAGE 26 vee Un The SUMMER JOBS 4 CAREER GUIDE ^Ooslv^ UWM THIS WEEK ONLY! April 30,2007 The student-run independent news weekly • Since 1956 Volume 51 | Issue 29 Every vote counts Students protest in the Union during elections. Post photo by Ryan Cardarella Students protest during SA elections Black Student Union protests election process, SAC decision By Ryan Cardarella Campus Government Editor "We should have been excused for a Members of the University of Wisconsin-Milwaukee Black death; everyone in our Student Union protested the organization was at the past week's Student Associa tion elections and a recent funeral." Senate Appropriations Com - Angelique Sharpe, mittee decision on Thursday in the UWM Union. Black Student Union Near the Union polling senior vice president area, protestors held signs illustrating their displeasure with the current administra tion. The protest was to be "I'm not going to held outside in Spaights Pla za, but heavy rainfall moved pander to emotions; it indoors. they didn't follow In a statement released by the Black Student Union the process and they (BSU), the demonstration was organized to protest shouldn't have been on "the amoral and unconstitu the agenda." tional actions of current SA - Tobin Huibregtse, Adam Tafoya, 23, submits his SA election ballot in the Union Thursday morning. Election results were not avail able as of press time on Sunday. Post photo by Kyle Stevens See PROTEST page 10 former SAC vice chair Carjacking occurs near Union UWM adds more online classes "When in that situation, in the fall semester.