University of Southampton Research Repository
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

North Carolina Wildlife Resources Commission Gordon Myers, Executive Director
North Carolina Wildlife Resources Commission Gordon Myers, Executive Director March 1, 2016 Honorable Jimmy Dixon Honorable Chuck McGrady N.C. House of Representatives N.C. House of Representatives 300 N. Salisbury Street, Room 416B 300 N. Salisbury Street, Room 304 Raleigh, NC 27603-5925 Raleigh, NC 27603-5925 Senator Trudy Wade N.C. Senate 300 N. Salisbury Street, Room 521 Raleigh, NC 27603-5925 Dear Honorables: I am submitting this report to the Environmental Review Committee in fulfillment of the requirements of Section 4.33 of Session Law 2015-286 (H765). As directed, this report includes a review of methods and criteria used by the NC Wildlife Resources Commission on the State protected animal list as defined in G.S. 113-331 and compares them to federal and state agencies in the region. This report also reviews North Carolina policies specific to introduced species along with determining recommendations for improvements to these policies among state and federally listed species as well as nonlisted animals. If you have questions or need additional information, please contact me by phone at (919) 707-0151 or via email at [email protected]. Sincerely, Gordon Myers Executive Director North Carolina Wildlife Resources Commission Report on Study Conducted Pursuant to S.L. 2015-286 To the Environmental Review Commission March 1, 2016 Section 4.33 of Session Law 2015-286 (H765) directed the N.C. Wildlife Resources Commission (WRC) to “review the methods and criteria by which it adds, removes, or changes the status of animals on the state protected animal list as defined in G.S. -

Information on the NCWRC's Scientific Council of Fishes Rare
A Summary of the 2010 Reevaluation of Status Listings for Jeopardized Freshwater Fishes in North Carolina Submitted by Bryn H. Tracy North Carolina Division of Water Resources North Carolina Department of Environment and Natural Resources Raleigh, NC On behalf of the NCWRC’s Scientific Council of Fishes November 01, 2014 Bigeye Jumprock, Scartomyzon (Moxostoma) ariommum, State Threatened Photograph by Noel Burkhead and Robert Jenkins, courtesy of the Virginia Division of Game and Inland Fisheries and the Southeastern Fishes Council (http://www.sefishescouncil.org/). Table of Contents Page Introduction......................................................................................................................................... 3 2010 Reevaluation of Status Listings for Jeopardized Freshwater Fishes In North Carolina ........... 4 Summaries from the 2010 Reevaluation of Status Listings for Jeopardized Freshwater Fishes in North Carolina .......................................................................................................................... 12 Recent Activities of NCWRC’s Scientific Council of Fishes .................................................. 13 North Carolina’s Imperiled Fish Fauna, Part I, Ohio Lamprey .............................................. 14 North Carolina’s Imperiled Fish Fauna, Part II, “Atlantic” Highfin Carpsucker ...................... 17 North Carolina’s Imperiled Fish Fauna, Part III, Tennessee Darter ...................................... 20 North Carolina’s Imperiled Fish Fauna, Part -

Endangered Species
FEATURE: ENDANGERED SPECIES Conservation Status of Imperiled North American Freshwater and Diadromous Fishes ABSTRACT: This is the third compilation of imperiled (i.e., endangered, threatened, vulnerable) plus extinct freshwater and diadromous fishes of North America prepared by the American Fisheries Society’s Endangered Species Committee. Since the last revision in 1989, imperilment of inland fishes has increased substantially. This list includes 700 extant taxa representing 133 genera and 36 families, a 92% increase over the 364 listed in 1989. The increase reflects the addition of distinct populations, previously non-imperiled fishes, and recently described or discovered taxa. Approximately 39% of described fish species of the continent are imperiled. There are 230 vulnerable, 190 threatened, and 280 endangered extant taxa, and 61 taxa presumed extinct or extirpated from nature. Of those that were imperiled in 1989, most (89%) are the same or worse in conservation status; only 6% have improved in status, and 5% were delisted for various reasons. Habitat degradation and nonindigenous species are the main threats to at-risk fishes, many of which are restricted to small ranges. Documenting the diversity and status of rare fishes is a critical step in identifying and implementing appropriate actions necessary for their protection and management. Howard L. Jelks, Frank McCormick, Stephen J. Walsh, Joseph S. Nelson, Noel M. Burkhead, Steven P. Platania, Salvador Contreras-Balderas, Brady A. Porter, Edmundo Díaz-Pardo, Claude B. Renaud, Dean A. Hendrickson, Juan Jacobo Schmitter-Soto, John Lyons, Eric B. Taylor, and Nicholas E. Mandrak, Melvin L. Warren, Jr. Jelks, Walsh, and Burkhead are research McCormick is a biologist with the biologists with the U.S. -

A Case Study of Carolina Bays and Ditched Streams at Risk Under the Proposed WOTUS Definition
CAPE FEAR RIVER WATERSHED: A Case Study of Carolina Bays and Ditched Streams at Risk under the Proposed WOTUS Definition The Cape Fear River. Photo by Kemp Burdette The Cape Fear River Basin is North Carolina’s largest watershed, with an area of over 9,000 square miles. Major tributaries include the Deep River, the Haw River, the Northeast Cape Fear River, the Black River, and the South River. These rivers converge to form a thirty-mile-long estuary before flowing into the Atlantic Ocean at Cape Fear.1 The Cape Fear supplies water to some of the fastest growing counties in the United States;2 roughly one in five North Carolinians gets their drinking water from the Cape Fear, including residents of Greensboro, Fayetteville, and Wilmington.3 The Cape Fear Basin is a popular watershed for a variety of recreation activities. State parks along the river include Haw River State Park, Raven Rock State Park, and Carolina Beach State Park. The faster-flowing water of the upper basin is popular with paddlers, as are the slow meandering blackwater rivers and streams of the lower Cape Fear and estuary. Fishing is very popular; the Cape Fear supports a number of freshwater species, saltwater species, and even anadromous (migratory) species like the endangered sturgeon, striped bass, and shad. Cape Fear River Watershed: Case Study Page 2 of 8 The Cape Fear is North Carolina’s most ecologically diverse watershed; the Lower Cape Fear is notable because it is part of a biodiversity “hotspot,” recording the largest degree of biodiversity on the eastern seaboard of the United States. -

Coastal Resilience Assessment of the Cape Fear Watershed
PILOT: Coastal Resilience Assessment of the Cape Fear Watershed i IMPORTANT INFORMATION/DISCLAIMER: This report represents a Regional Coastal Resilience Assessment that can be used to identify places on the landscape for resilience-building efforts and conservation actions through understanding coastal flood threats, the exposure of populations and infrastructure have to those threats, and the presence of suitable fish and wildlife habitat. As with all remotely sensed or publicly available data, all features should be verified with a site visit, as the locations of suitable landscapes or areas containing flood hazards and community assets are approximate. The data, maps, and analysis provided should be used only as a screening-level resource to support management decisions. This report should be used strictly as a planning reference tool and not for permitting or other legal purposes. The scientific results and conclusions, as well as any views or opinions expressed herein, are those of the authors and should not be interpreted as representing the opinions or policies of the U.S. Government, or the National Fish and Wildlife Foundation’s partners. Mention of trade names or commercial products does not constitute their endorsement by the U.S. Government or the National Fish and Wildlife Foundation or its funding sources. NATIONAL OCEANIC AND ATMOSPHERIC ADMINISTRATION DISCLAIMER: The scientific results and conclusions, as well as any views or opinions expressed herein, are those of the author(s) and do not necessarily reflect those of NOAA or the Department of Commerce. U.S. ARMY CORPS OF ENGINEERS DISCLAIMER: NFWF’s assessment methodology focuses on identifying and ranking Resilience Hubs, or undeveloped areas of open space. -

Aquatic Species Mapping in North Carolina Using Maxent
Aquatic Species Mapping in North Carolina Using Maxent Mark Endries U.S. Fish and Wildlife Service, Ecological Services Field Office, Asheville North Carolina INTRODUCTION The mission of the U.S. Fish and Wildlife Service (Service) is to work with others to conserve, protect, and enhance fish, wildlife, and plants and their habitats for the continuing benefit of the American people. The Service is the lead governmental agency involved in the recovery of federally endangered and threatened species in freshwater and terrestrial habitats. To meet its recovery and protection goals, the Service: (1) works with other federal agencies to minimize or eliminate impacts to fish, wildlife, and plants from projects they authorize, fund, or carry out; (2) supports the improvement of fish and wildlife habitat on private land through technical and financial assistance; and (3) provides scientific knowledge and analyses to help guide the conservation, development, and management of the Nation’s fish and wildlife resources. Freshwater ecosystems present unique management challenges due to their linear spatial orientation and their association with upland habitat variables. On broad scales, the movement of aquatic species within the stream environment is limited to upstream and downstream migration. The inability of aquatic species to circumnavigate man-made obstacles causes them to be particularly vulnerable to habitat fragmentation. Habitat fragmentation has a major influence on species distribution and complicates distribution mapping. To better understand the spatial distributions of freshwater aquatic species in North Carolina, the Service created predictive habitat maps for 226 different aquatic species using geographic information systems (GIS) and maximum entropy (Maxent) modeling. These maps were derived by comparing known species occurrences with a suite of stream- or land-cover-derived environmental variables. -
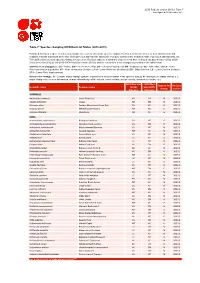
Table 7: Species Changing IUCN Red List Status (2012-2013)
IUCN Red List version 2013.2: Table 7 Last Updated: 25 November 2013 Table 7: Species changing IUCN Red List Status (2012-2013) Published listings of a species' status may change for a variety of reasons (genuine improvement or deterioration in status; new information being available that was not known at the time of the previous assessment; taxonomic changes; corrections to mistakes made in previous assessments, etc. To help Red List users interpret the changes between the Red List updates, a summary of species that have changed category between 2012 (IUCN Red List version 2012.2) and 2013 (IUCN Red List version 2013.2) and the reasons for these changes is provided in the table below. IUCN Red List Categories: EX - Extinct, EW - Extinct in the Wild, CR - Critically Endangered, EN - Endangered, VU - Vulnerable, LR/cd - Lower Risk/conservation dependent, NT - Near Threatened (includes LR/nt - Lower Risk/near threatened), DD - Data Deficient, LC - Least Concern (includes LR/lc - Lower Risk, least concern). Reasons for change: G - Genuine status change (genuine improvement or deterioration in the species' status); N - Non-genuine status change (i.e., status changes due to new information, improved knowledge of the criteria, incorrect data used previously, taxonomic revision, etc.) IUCN Red List IUCN Red Reason for Red List Scientific name Common name (2012) List (2013) change version Category Category MAMMALS Nycticebus javanicus Javan Slow Loris EN CR N 2013.2 Okapia johnstoni Okapi NT EN N 2013.2 Pteropus niger Greater Mascarene Flying -

Notropis Mekistocholas, a New Herbivorous Cyprinid Fish ~Ndernic to the Cape Fear River Basin, North Carolina
Notropis mekistocholas, a New Herbivorous Cyprinid Fish ~ndernic to the Cape Fear River Basin, North Carolina FRA1"lKLIN F. SNELSON, JR. Notropis mekistocholas is described as a new species. It is unusual within the genus in exhibiting herbivorous adaptations-an elongate, convoluted intestine and black peritoneum. It is compared with N. alborus and N. procne, two sympatric relatives that differ in lacking herbivorous modifications, in hav.ing seven rather than eight anal rays, and in numerous other features. N. mekistocholas is the first known endemic species from the Cape Fear drainage in North Carolina. It has a very restricted distribution in the east-central Piedmont province, being known from only four streams in Chatham and Harnett counties. Intestinal modifications suggestive of an herbivorous diet are reported for N. anogenus and some species of the subgenus Luxilus. There is no evidence to suggest that N. mekistocholas should be aligned with the south. western genus Dionda, which contains herbivorous species superifically similar to Notropis species. Closest relatives of N. mekistocholas appear to be N. procne and N. stramineus. INTRODUCTION The number of predorsal scale rows in- HE genus NotroPis usually has been char. cludes all oblique scale rows crossing an imaginary line between the anterior inser- T acterized as having a short, S-shaped ali- mentary tract, distinguishing it from closely tion of the dorsal fin and the cleithrum. The related groups containing herbivorous forms first row counted was the one including the with elongate intestines. Discovery of a first predorsal scale; the last row counted was unique new species of Notropis, closely re- the one including the first (anterior-most) lated to N. -

Positive Interactions in Freshwater Systems
Clemson University TigerPrints All Theses Theses 12-2018 Positive Interactions in Freshwater Systems Samuel Clarence Silknetter Clemson University, [email protected] Follow this and additional works at: https://tigerprints.clemson.edu/all_theses Recommended Citation Silknetter, Samuel Clarence, "Positive Interactions in Freshwater Systems" (2018). All Theses. 3257. https://tigerprints.clemson.edu/all_theses/3257 This Thesis is brought to you for free and open access by the Theses at TigerPrints. It has been accepted for inclusion in All Theses by an authorized administrator of TigerPrints. For more information, please contact [email protected]. POSITIVE INTERACTIONS IN FRESHWATER SYSTEMS A Thesis Presented to the Graduate School of Clemson University In Partial Fulfillment of the Requirements for the Degree Master of Science Wildlife and Fisheries Biology by Samuel Clarence Silknetter December 2018 Accepted by: Dr. Brandon Peoples, Committee Chair Dr. Kyle Barrett Dr. Troy Farmer ABSTRACT The goals of this thesis are twofold, 1) to review the existing literature on positive interactions, and 2) to experimentally test the role of biotic context in a freshwater reproductive interaction. First, my co-authors and I have conducted a review of positive interactions in freshwaters to establish a direction for future research. By outlining case studies and causal mechanisms, we illustrate the diversity of positive interactions, and set the stage for a comprehensive look at the role of context in shaping interaction outcomes. Our research directive focuses on the value of both basic life history and experimental research, then using those findings to investigate these interactions under different contexts, in different levels of ecosystem structure, and at multiple geographic scales. -

Notropis Mekistocholas)
HABITAT USE OF LARVAL AND JUVENILE CAPE FEAR SHINERS (NOTROPIS MEKISTOCHOLAS) Except where reference is made to the work of others, the work described in this thesis is my own or was done in collaboration with my advisory committee. This thesis does not include proprietary or classified information. _________________________________ Andrew Reid Henderson Certificate of Approval: ____________________________ _____________________________ Carol E. Johnston, Chair Mary C. Freeman Associate Professor Affiliate Professor Fisheries and Allied Aquacultures Fisheries and Allied Aquacultures ____________________________ _____________________________ Craig Guyer George T. Flowers Professor Dean Biological Sciences Graduate School HABITAT USE OF LARVAL AND JUVENILE CAPE FEAR SHINERS (NOTROPIS MEKISTOCHOLAS) Andrew Reid Henderson A Thesis Submitted to the Graduate Faculty of Auburn University in Partial Fulfillment of the Degree of Master of Science Auburn, AL December 19, 2008 HABITAT USE OF LARVAL AND JUVENILE CAPE FEAR SHINERS (NOTROPIS MEKISTOCHOLAS) Permission is granted to Auburn University to make copies of this thesis at its discretion, upon request of individuals or institutions and at their expense. The author reserves all publication rights. ______________________________ Signature of Author ______________________________ Date of Graduation iii VITA Andrew Reid Henderson was born in Birmingham, AL, on July 25, 1980, and raised in Springville, Alabama. He attended Auburn University and graduated with a bachelor's degree in Fisheries and Allied Aquacultures in 2004. After graduation, he worked as an intern for the Stream Survey Team of the Georgia Department of Natural Resources. In July 2005 he began graduate school in the Department of Fisheries and Allied Aquacultures. iv THESIS ABSTRACT HABITAT USE OF LARVAL AND JUVENILE CAPE FEAR SHINERS (NOTROPIS MEKISTOCHOLAS) Andrew R. -

Partnerships in Conservation for Central and Eastern North Carolina
U.S. Fish and Wildlife Service Partnerships in Conservation 2018 for Central and Eastern North Carolina Raleigh Field Office ur 2018 conservation work is in full swing We are working with our partners in federal and with follow-through from previous years state agencies, local governments, the business Oand new conservation ventures. Raleigh community, and private citizens in 59 counties of Ecological Services Field Office staff are dedicat- central and eastern North Carolina. Together, ed to using the best available science to further we are protecting important habitat, increasing the U.S. Fish and Wildlife Service’s (Service) species’ populations, and reducing the threats to mission. We seek to conserve imperiled species their survival. Our partners are pivotal and share and their habitats, ensuring that sustainable in all of the following conservation updates and populations of fish, wildlife, and plants continue accomplishments. to thrive for future generations. Contents Identifying species on the verge of extinction - Helping build the road to recovery to bring them back - Providing technical assistance to improve fish and wildlife outcomes - Employing scientific excellence to support partners’ needs - Partnering with others to restore fish and wildlife habitats -- Developing a workforce of conservation leaders River waterdog and the Atlantic pigtoe in 2018. American eel age and growth assessment in Advancing conservation science for the car- Simultaneously, we continue to pursue voluntary the Roanoke River nivorous Venus flytrap conservation measures and partnerships for at- Identifying species on the risk species. For example, we are working with he Service reviewed the status of American the National Fish and Wildlife Foundation, the eels in 2007 and in 2015, finding both times NCWRC, Conservation Fisheries, Inc. -

Conservation Genetics of Five Species of Dionda in West Texas
CONSERVATION GENETICS OF FIVE SPECIES OF DIONDA IN WEST TEXAS A Thesis by ASHLEY HELEN HANNA Submitted to the Office of Graduate Studies of Texas A&M University in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE December 2011 Major Subject: Wildlife and Fisheries Sciences Conservation Genetics of Five Species of Dionda in West Texas Copyright 2011 Ashley Helen Hanna CONSERVATION GENETICS OF FIVE SPECIES OF DIONDA IN WEST TEXAS A Thesis by ASHLEY HELEN HANNA Submitted to the Office of Graduate Studies of Texas A&M University in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE Approved by: Chair of Committee, John R. Gold Committee Members, J. Spencer Johnston R. Neal Wilkins Head of Department, John B. Carey December 2011 Major Subject: Wildlife and Fisheries Sciences iii ABSTRACT Conservation Genetics of Five Species of Dionda in West Texas. (December 2011) Ashley Helen Hanna Chair of Advisory Committee: Dr. John R. Gold Minnows of the genus Dionda (Cyprinidae, Teleostei) inhabit spring-fed streams in the southwestern United States and Mexico. Five nominal species of Dionda (D. argentosa, D. diaboli, D. episcopa, D. nigrotaeniata and D. serena) are found in streams and rivers in central and west Texas. Because Dionda require clean, flowing water, they serve as aquatic indicator species of biological impacts of drought and human water use. Consequently, the ecological and conservation status of species of Dionda are important relative to monitoring habitat deterioration. This study used genetic data from geographic samples of the five nominal species of Dionda in Texas waters to document the conservation-genetics status of populations in each species.