The Role of Surgery in Optic Pathway/Hypothalamic Gliomas in Children
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Isolated Relative Afferent Pupillary Defect Secondary to Contralateral Midbrain Compression
OBSERVATION Isolated Relative Afferent Pupillary Defect Secondary to Contralateral Midbrain Compression Cheun Ju Chen, MD; Mia Scheufele, MD; Maushmi Sheth, MD; Amir Torabi, MD; Nick Hogan, MD, PhD; Elliot M. Frohman, MD, PhD Background: Relative afferent pupillary defects are typi- accounts for the relative afferent pupillary defect con- cally related to ipsilateral lesions within the anterior vi- tralateral to the described lesion. sual pathways. Result: Magnetic resonance imaging of the brain revealed a pineal tumor compressing the right rostral midbrain. Objective: To describe a patient who had a workup for headache and was found to have an isolated left relative Conclusion: While rare, a relative afferent pupillary de- afferent pupillary defect without any other neurological fect can occasionally occur secondary to lesions in the findings. postchiasmal pathways. In these circumstances, the pu- pillary defect will be observed to be contralateral to the Design: We review the neuroanatomy of the pupil- side of the lesion. lary light reflex pathway and emphasize the nasotem- poral bias of decussating fiber projections, which Arch Neurol. 2004;61:1451-1453 RELATIVE AFFERENT PUPIL- though retinal fibers concerned with this lary defect (RAPD) is char- reflex transmit information to both the ip- acterized by pupillary dila- silateral and contralateral midbrain, there tion upon illuminating the is a slight crossing bias, with about 53% of eye during the swinging the fibers crossing in the optic chiasm Aflashlight test. The presence of this sign sig- (chiefly derived from the nasal retina) and nifies an abnormality in the transmission 47% remaining ipsilateral. This anatomi- of light information within the pupillary cal organization of the pupillary constric- light constrictor pathway from the retina tor pathway results in the possibility of pro- to the rostral midbrain circuitry involved ducing an RAPD during illumination of the in this reflex. -

Measurement of the Normal Optic Chiasm on Coronal MR Images
Measurement of the Normal Optic Chiasm on Coronal MR Images Andrew L. Wagner, F. Reed Murtagh, Ken S. Hazlett, and John A. Arrington PURPOSE: To develop an objective method for measuring the optic chiasm and to document its normal range in size. METHODS: Measurements of the height and area of the optic chiasm, made on coronal T1-weighted MR images with the use of commercially available region-of-interest software, were obtained in 114 healthy subjects who had a total of 123 MR studies. A normal range and standard deviation were calculated, and the information was broken down by age and sex. RESULTS: The mean area of the optic chiasm was 43.7 mm2, with a standard deviation of 5.21. The mean width was 14.0 mm, with a standard deviation of 1.68. CONCLUSION: The area and width of the optic chiasm can be measured with the use of commercially available software, which allows an objective estimate of the chiasm’s size. Knowledge of the normal size range of the optic chiasm can be helpful in the early detection of some disorders. Index terms: Optic chiasm; Brain, anatomy; Brain, measurement AJNR Am J Neuroradiol 18:723–726, April 1997 The optic chiasm is an important land- months and that had been interpreted as normal. No pa- mark when interpreting magnetic resonance (MR) tient had suspected visual or endocrine abnormalities. All examinations of the brain. A small chiasm can be the examinations had been performed with a 1.5-T Gen- an indication of several disorders, the most com- eral Electric (Milwaukee, Wis) Signa or 1.5-T Siemens mon of which is septooptic dysplasia (1), and a (Cary, NC) Somatom MR system using routine imaging large chiasm can be the result of glioma, menin- protocols, with additional 3-mm T1-weighted contiguous coronal sections used for measurements. -
A Case of Intramedullary Spinal Cord Astrocytoma Associated with Neurofibromatosis Type 1
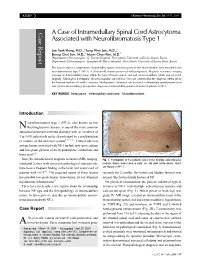
KISEP J Korean Neurosurg Soc 36 : 69-71, 2004 Case Report A Case of Intramedullary Spinal Cord Astrocytoma Associated with Neurofibromatosis Type 1 Jae Taek Hong, M.D.,1 Sang Won Lee, M.D.,1 Byung Chul Son, M.D.,1 Moon Chan Kim, M.D.2 Department of Neurosurgery,1 St. Vincent Hospital, The Catholic University of Korea, Suwon, Korea Department of Neurosurgery,2 Kangnam St. Mary's Hospital, The Catholic University of Korea, Seoul, Korea The authors report a symptomatic intramedullary spinal cord astrocytoma in the thoracolumbar area associated with neurofibromatosis type 1 (NF-1). A 38-year-old woman presented with paraparesis. Magnetic resonance imaging revealed an intramedullary lesion within the lower thoracic spinal cord and conus medullaris, which was removed surgically. Pathological investigation showed anaplastic astrocytoma. This case confirms that the diagnosis criteria set by the National Institute of Health Consensus Development Conference can be useful to differentiate ependymoma from astrocytoma when making a preoperative diagnosis of intramedullary spinal cord tumor in patients of NF-1. KEY WORDS : Astrocytoma·Intramedullary cord tumor·Neurofibromatosis. Introduction eurofibromatosis type 1 (NF-1), also known as von N Recklinghausen's disease, is one of the most common autosomal dominant inherited disorders with an incidence of 1 in 3,000 individuals and is characterized by a predisposition to tumors of the nervous system5,6,12,16). Central nervous system lesions associated with NF-1 include optic nerve glioma and low-grade gliomas of the hypothalamus, cerebellum and brain stem6,10). Since the introduction of magnetic resonance(MR) imaging, Fig. 1. Photograph of the patient's back shows multiple subcutaneous incidental lesions with uncertain pathological characteristic nodules (black arrow) and a cafe-au-lait spot (white arrow), which have been a frequent finding in the brain and spinal cord of are typical of NF-1. -

Pediatric Orbital Tumors and Lacrimal Drainage System
Pediatric Orbital Tumors and Lacrimal Drainage System Peter MacIntosh, MD University of Illinois • No financial disclosures Dermoid Cyst • Congenital • Keratinized epidermis • Dermal appendage • Trapped during embryogenesis • 6% of lesions • 40-50% of orbital pediatric orbital lesion • Usually discovered in the first year of life • Painless/firm/subQ mass • Rarely presents as an acute inflammatory lesion (Rupture?) • Frontozygomatic (70%) • Maxillofrontal (20%) suture Imaging - CT • Erosion/remodeling of bone • Adjacent bony changes: “smooth fossa” (85%) • Dumbell dermoid: extraorbital and intraorbital components through bony defect Imaging - MRI • Encapsulated • Enhancement of wall but not lumen Treatment Options • Observation • Risk of anesthesia • Surgical Removal • Changes to bone • Rupture of cyst can lead to acute inflammation • Irrigation • Abx • Steroids Dermoid INFANTILE/Capillary Hemangioma • Common BENIGN orbital lesion of children • F>M • Prematurity • Appears in 1st or 2nd week of life • Soft, bluish mass deep to the eyelid • Superonasal orbit • Rapidly expands over 6-12 months • Increases with valsalva (crying) • Clinical findings • Proptosis Astigmatism • Strabismus Amblyopia INFANTILE/Capillary Hemangioma • May enlarge for 1-2 years then regress • 70-80% resolve before age 7 • HIGH flow on doppler • Kasabach-Merritt Syndrome • Multiple large visceral capillary hemangiomas • Sequestration of platelets into tumor • Consumptive thrombocytopenia • Supportive therapy and treat underlying tumor • Complications • DIC • death •Homogenous -

Neuro-Oncology 1 XX(XX), 1–12, 2016 | Doi:10.1093/Neuonc/Now267
Neuro-Oncology 1 XX(XX), 1–12, 2016 | doi:10.1093/neuonc/now267 Defining the temporal course of murine neurofibromatosis-1 optic gliomagenesis reveals a therapeutic window to attenuate retinal dysfunction Joseph A. Toonen, Yu Ma, and David H. Gutmann Department of Neurology, Washington University School of Medicine (WUSM), St Louis, Missouri (J.A.T., Y.M., D.H.G.) Corresponding Author: David H. Gutmann, MD, PhD, Department of Neurology, Washington University, Box 8111, 660 S. Euclid Avenue, St. Louis MO 63110 ([email protected]). Abstract Background. Optic gliomas arising in the neurofibromatosis type 1 (NF1) cancer predisposition syndrome cause reduced visual acuity in 30%–50% of affected children. Since human specimens are rare, genetically engineered mouse (GEM) models have been successfully employed for preclinical therapeutic discovery and validation. However, the sequence of cellular and molecular events that culminate in retinal dysfunction and vision loss has not been fully defined relevant to potential neuroprotective treatment strategies. Methods. Nf1flox/mut GFAP-Cre (FMC) mice and age-matched Nf1flox/flox (FF) controls were euthanized at defined intervals from 2 weeks to 24 weeks of age. Optic nerve volumes were measured, and optic nerves/retinae analyzed by immunohistochemistry. Optical coherence tomography (OCT) was performed on anesthetized mice. FMC mice were treated with lovastatin from 12 to 16 weeks of age. Results. The earliest event in tumorigenesis was a persistent elevation in proliferation (4 wk), which preceded sustained microglia numbers and incremental increases in S100+ glial cells. Microglia activation, as evidenced by increased interleukin (IL)-1β expression and morphologic changes, coincided with axonal injury and retinal ganglion cell (RGC) apoptosis (6 wk). -

Clinical Manifestations of Hypothalamic Tumors*
ANNALS OF CLINICAL AND LABORATORY SCIENCE, Vol. 10, No. 6 Copyright © 1980, Institute for Clinical Science, Inc. Clinical Manifestations of Hypothalamic Tumors* ADOLFO D. GARNICA, M.D., MICHAEL L. NETZLOFF, M.D.,f and A. L. ROSENBLOOM, M.D. Department of Pediatrics, University of Florida College of Medicine, Gainesville, FL 32610 and f Department of Human Development, Michigan State University East Lansing, MI 88823 ABSTRACT The regulatory function of the central nervous system encompasses di verse endocrine, metabolic, and behavioral processes. Many of these origi nate, are integrated, or are coordinated through hypothalamic pathways or nuclei. Thus, tumors affecting areas projecting to the hypothalamus, tumors of the hypothalamus, and tumors invading or compressing the hypothalamus can produce abnormalities of hypothalamic function. Introduction tary.4,7,31 A secretory function for certain hypothalamic neurons was postulated in Until recently, no endocrine disorder 1928 and subsequently confirmed by the directly attributable to hypothalamic dys demonstration of hormone synthesis in function had been recognized, and the the supraoptic and paraventricular nu majority of endocrine-metabolic homeo clei.28,53 Moreover, observations on the static processes were acknowledged to be effects of environment on the menstrual under the control of the anterior pitui cycles of women and the study of repro tary.48,49 However, in 1901 Frohlich re ductive cycles in animals have shown a ported a patient with a suprasellar tumor, functional connection -

Anatomy and Physiology of the Afferent Visual System
Handbook of Clinical Neurology, Vol. 102 (3rd series) Neuro-ophthalmology C. Kennard and R.J. Leigh, Editors # 2011 Elsevier B.V. All rights reserved Chapter 1 Anatomy and physiology of the afferent visual system SASHANK PRASAD 1* AND STEVEN L. GALETTA 2 1Division of Neuro-ophthalmology, Department of Neurology, Brigham and Womens Hospital, Harvard Medical School, Boston, MA, USA 2Neuro-ophthalmology Division, Department of Neurology, Hospital of the University of Pennsylvania, Philadelphia, PA, USA INTRODUCTION light without distortion (Maurice, 1970). The tear–air interface and cornea contribute more to the focusing Visual processing poses an enormous computational of light than the lens does; unlike the lens, however, the challenge for the brain, which has evolved highly focusing power of the cornea is fixed. The ciliary mus- organized and efficient neural systems to meet these cles dynamically adjust the shape of the lens in order demands. In primates, approximately 55% of the cortex to focus light optimally from varying distances upon is specialized for visual processing (compared to 3% for the retina (accommodation). The total amount of light auditory processing and 11% for somatosensory pro- reaching the retina is controlled by regulation of the cessing) (Felleman and Van Essen, 1991). Over the past pupil aperture. Ultimately, the visual image becomes several decades there has been an explosion in scientific projected upside-down and backwards on to the retina understanding of these complex pathways and net- (Fishman, 1973). works. Detailed knowledge of the anatomy of the visual The majority of the blood supply to structures of the system, in combination with skilled examination, allows eye arrives via the ophthalmic artery, which is the first precise localization of neuropathological processes. -

1. Lateral View of Lobes in Left Hemisphere TOPOGRAPHY
TOPOGRAPHY T1 Division of Cerebral Cortex into Lobes 1. Lateral View of Lobes in Left Hemisphere 2. Medial View of Lobes in Right Hemisphere PARIETAL PARIETAL LIMBIC FRONTAL FRONTAL INSULAR: buried OCCIPITAL OCCIPITAL in lateral fissure TEMPORAL TEMPORAL 3. Dorsal View of Lobes 4. Ventral View of Lobes PARIETAL TEMPORAL LIMBIC FRONTAL OCCIPITAL FRONTAL OCCIPITAL Comment: The cerebral lobes are arbitrary divisions of the cerebrum, taking their names, for the most part, from overlying bones. They are not functional subdivisions of the brain, but serve as a reference for locating specific functions within them. The anterior (rostral) end of the frontal lobe is referred to as the frontal pole. Similarly, the anterior end of the temporal lobe is the temporal pole, and the posterior end of the occipital lobe the occipital pole. TOPOGRAPHY T2 central sulcus central sulcus parietal frontal occipital lateral temporal lateral sulcus sulcus SUMMARY CARTOON: LOBES SUMMARY CARTOON: GYRI Lateral View of Left Hemisphere central sulcus postcentral superior parietal superior precentral gyrus gyrus lobule frontal intraparietal sulcus gyrus inferior parietal lobule: supramarginal and angular gyri middle frontal parieto-occipital sulcus gyrus incision for close-up below OP T preoccipital O notch inferior frontal cerebellum gyrus: O-orbital lateral T-triangular sulcus superior, middle and inferior temporal gyri OP-opercular Lateral View of Insula central sulcus cut surface corresponding to incision in above figure insula superior temporal gyrus Comment: Insula (insular gyri) exposed by removal of overlying opercula (“lids” of frontal and parietal cortex). TOPOGRAPHY T3 Language sites and arcuate fasciculus. MRI reconstruction from a volunteer. central sulcus supramarginal site (posterior Wernicke’s) Language sites (squares) approximated from electrical stimulation sites in patients undergoing operations for epilepsy or tumor removal (Ojeman and Berger). -

Cranial Nerves II, III, IV & VI (Optic, Oculomotor, Trochlear, & Abducens)
Cranial Nerves II, III, IV & VI (Optic, Oculomotor, Trochlear, & Abducens) Lecture (13) ▪ Important ▪ Doctors Notes Please check our Editing File ▪ Notes/Extra explanation ه هذا العمل مب ين بشكل أسا يس عىل عمل دفعة 436 مع المراجعة { َوَم نْ يَ َت َو َ ّكْ عَ َلْ ا َّْلل فَهُ َوْ َحْ سْ ُ ُُْ} والتدقيق وإضافة المﻻحظات وﻻ يغ ين عن المصدر اﻷسا يس للمذاكرة ▪ Objectives At the end of the lecture, students should be able to: ✓ List the cranial nuclei related to occulomotor, trochlear, and abducent nerves in the brain stem. ✓ Describe the type and site of each nucleus. ✓ Describe the site of emergence and course of these 3 nerves. ✓ Describe the important relations of oculomotor, trochlear, and abducent nerves in the orbit ✓ List the orbital muscles supplied by each of these 3 nerves. ✓ Describe the effect of lesion of each of these 3 nerves. ✓ Describe the optic nerve and visual pathway. Recall the how these nerves exit from the brain stem: Optic (does not exit from brain stem) Occulomotor: ventral midbrain (medial aspect of crus cerebri) Trochlear: dorsal midbrain (caudal to inferior colliculus) Abducent: ventral Pons (junction b/w pons & pyramid) Brain (Ventral view) Brain stem (Lateral view) Extra-Ocular Muscles 7 muscles: (ترفع جفن العين) .Levator palpebrae superioris 1- Origin: from the roof of the orbit (4) Recti muscles: *Rectus: ماشي على ( Superior rectus (upward and medially 2- الصراط (Inferior rectus (downward and medially 3- المستقيم 4- Medial rectus (medial) (medial) 5- Lateral rectus (lateral) How to remember the 2 فحركته muscles not supplied by نفس اسمه -اسمها عكس وظيفتها- :Oblique muscles (2) 6- Superior oblique (downward and laterally) Oblique: CN3? Superior oblique goes -1 منحرفOrigin: from the roof of the orbit 7- Inferior oblique (upward and laterally) up (superior) and turns around (oblique) a notch يمشي Origin: from the anterior floor or pulley and its supply is عكس كﻻمه NB. -

Spectrum of Clinical and Associated MR Imaging Findings in Children with Olfactory Anomalies
Published March 17, 2016 as 10.3174/ajnr.A4738 ORIGINAL RESEARCH PEDIATRICS Spectrum of Clinical and Associated MR Imaging Findings in Children with Olfactory Anomalies X T.N. Booth and X N.K. Rollins ABSTRACT BACKGROUND AND PURPOSE: The olfactory apparatus, consisting of the bulb and tract, is readily identifiable on MR imaging. Anom- alous development of the olfactory apparatus may be the harbinger of anomalies of the secondary olfactory cortex and associated structures. We report a large single-site series of associated MR imaging findings in patients with olfactory anomalies. MATERIALS AND METHODS: A retrospective search of radiologic reports (2010 through 2014) was performed by using the keyword “olfactory”; MR imaging studies were reviewed for olfactory anomalies and intracranial and skull base malformations. Medical records were reviewed for clinical symptoms, neuroendocrine dysfunction, syndromic associations, and genetics. RESULTS: We identified 41 patients with olfactory anomalies (range, 0.03–18 years of age; M/F ratio, 19:22); olfactory anomalies were bilateral in 31 of 41 patients (76%) and absent olfactory bulbs and olfactory tracts were found in 56 of 82 (68%). Developmental delay was found in 24 (59%), and seizures, in 14 (34%). Pituitary dysfunction was present in 14 (34%), 8 had panhypopituitarism, and 2 had isolated hypogonadotropic hypogonadism. CNS anomalies, seen in 95% of patients, included hippocampal dysplasia in 26, cortical malformations in 15, malformed corpus callosum in 10, and optic pathway hypoplasia in 12. Infratentorial anomalies were seen in 15 (37%) patients and included an abnormal brain stem in 9 and an abnormal cerebellum in 3. Four patients had an abnormal membranous labyrinth. -

Low-Grade Central Nervous System Tumors
Neurosurg Focus 12 (2):Article 1, 2002, Click here to return to Table of Contents Low-grade central nervous system tumors M. BEATRIZ S. LOPES, M.D., AND EDWARD R. LAWS, JR., M.D. Departments of Pathology (Neuropathology) and Neurological Surgery, University of Virginia Health Sciences Center, Charlottesville, Virginia Low-grade tumors of the central nervous system constitute 15 to 35% of primary brain tumors. Although this cate- gory of tumors encompasses a number of different well-characterized entities, low-grade tumors constitute every tumor not obviously malignant at initial diagnosis. In this brief review, the authors discuss the pathological classification, diagnostic procedures, treatment, and possible pathogenic mechanisms of these tumors. Emphasis is given in the neu- roradiological and pathological features of the several entities. KEY WORDS • glioma • astrocytoma • treatment outcome Low-grade gliomas of the brain represent a large pro- toses. The pilocytic (juvenile) astrocytoma is a character- portion of primary brain tumors, ranging from 15 to 35% istic, more circumscribed lesion occurring primarily in in most reported series.1–5 They include a remarkable di- childhood and with a predilection for being located in the versity of lesions, all of which have been lumped together cerebellum. It usually appears as a cystic tumor with a under the heading of "low-grade glioma." This category mural nodule. The tumor tissue itself may have features of includes virtually every tumor of glial origin that is not microcystic degeneration and Rosenthal fibers which are overtly malignant at the time of initial diagnosis. degenerative structures in the astrocytic processes. Other reasonably common types of low-grade gliomas include CLASSIFICATION OF GLIOMAS the low-grade oligodendroglioma and the low-grade ependymoma, which is usually anatomically related to the Table 1 provides a classification of low-grade tumors of ventricular ependymal lining. -

Cranial Nerve Disorders: Clinical Manifestations and Topographyଝ
Radiología. 2019;61(2):99---123 www.elsevier.es/rx UPDATE IN RADIOLOGY Cranial nerve disorders: Clinical manifestations and topographyଝ a,∗ a b c M. Jorquera Moya , S. Merino Menéndez , J. Porta Etessam , J. Escribano Vera , a M. Yus Fuertes a Sección de Neurorradiología, Hospital Clínico San Carlos, Madrid, Spain b Servicio de Neurología, Hospital Clínico San Carlos, Madrid, Spain c Neurorradiología, Hospital Ruber Internacional, Madrid, Spain Received 17 November 2017; accepted 27 September 2018 KEYWORDS Abstract The detection of pathological conditions related to the twelve cranial pairs rep- Cranial pairs; resents a significant challenge for both clinicians and radiologists; imaging techniques are Cranial nerves; fundamental for the management of many patients with these conditions. In addition to knowl- Cranial neuropathies; edge about the anatomy and pathological entities that can potentially affect the cranial pairs, Neuralgia; the imaging evaluation of patients with possible cranial pair disorders requires specific exami- Cranial nerve palsy nation protocols, acquisition techniques, and image processing. This article provides a review of the most common symptoms and syndromes related with the cranial pairs that might require imaging tests, together with a brief overview of the anatomy, the most common underlying processes, and the most appropriate imaging tests for different indications. © 2018 SERAM. Published by Elsevier Espana,˜ S.L.U. All rights reserved. PALABRAS CLAVE Sintomatología derivada de los pares craneales: Clínica y topografía Pares craneales; Resumen La detección de la patología relacionada con los doce pares craneales representa Nervios craneales; un importante desafío, tanto para los clínicos como para los radiólogos. Las técnicas de imagen Neuropatía de pares craneales; son fundamentales para el manejo de muchos de los pacientes.