Body-Fluid Diagnostics in Microliter Samples
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

07 Chapter2.Pdf
22 METHODOLOGY 2.1 INTRODUCTION TO ELECTROCHEMICAL TECHNIQUES Electrochemical techniques of analysis involve the measurement of voltage or current. Such methods are concerned with the interplay between solution/electrode interfaces. The methods involve the changes of current, potential and charge as a function of chemical reactions. One or more of the four parameters i.e. potential, current, charge and time can be measured in these techniques and by plotting the graphs of these different parameters in various ways, one can get the desired information. Sensitivity, short analysis time, wide range of temperature, simplicity, use of many solvents are some of the advantages of these methods over the others which makes them useful in kinetic and thermodynamic studies1-3. In general, three electrodes viz., working electrode, the reference electrode, and the counter or auxiliary electrode are used for the measurement in electrochemical techniques. Depending on the combinations of parameters and types of electrodes there are various electrochemical techniques. These include potentiometry, polarography, voltammetry, cyclic voltammetry, chronopotentiometry, linear sweep techniques, amperometry, pulsed techniques etc. These techniques are mainly classified into static and dynamic methods. Static methods are those in which no current passes through the electrode-solution interface and the concentration of analyte species remains constant as in potentiometry. In dynamic methods, a current flows across the electrode-solution interface and the concentration of species changes such as in voltammetry and coulometry4. 2.2 VOLTAMMETRY The field of voltammetry was developed from polarography, which was invented by the Czechoslovakian Chemist Jaroslav Heyrovsky in the early 1920s5. Voltammetry is an electrochemical technique of analysis which includes the measurement of current as a function of applied potential under the conditions that promote polarization of working electrode6. -

Hydrodynamic Voltammetry As a Rapid and Simple Method for Evaluating Soil Enzyme Activities
Sensors 2015, 15, 5331-5343; doi:10.3390/s150305331 OPEN ACCESS sensors ISSN 1424-8220 www.mdpi.com/journal/sensors Article Hydrodynamic Voltammetry as a Rapid and Simple Method for Evaluating Soil Enzyme Activities Kazuto Sazawa 1,* and Hideki Kuramitz 2 1 Center for Far Eastern Studies, University of Toyama, Gofuku 3190, 930-8555 Toyama, Japan 2 Department of Environmental Biology and Chemistry, Graduate School of Science and Engineering for Research, University of Toyama, Gofuku 3190, 930-8555 Toyama, Japan; E-Mail: [email protected] * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel./Fax: +81-76-445-66-69. Academic Editor: Ki-Hyun Kim Received: 26 December 2014 / Accepted: 28 February 2015 / Published: 4 March 2015 Abstract: Soil enzymes play essential roles in catalyzing reactions necessary for nutrient cycling in the biosphere. They are also sensitive indicators of ecosystem stress, therefore their evaluation is very important in assessing soil health and quality. The standard soil enzyme assay method based on spectroscopic detection is a complicated operation that requires the removal of soil particles. The purpose of this study was to develop a new soil enzyme assay based on hydrodynamic electrochemical detection using a rotating disk electrode in a microliter droplet. The activities of enzymes were determined by measuring the electrochemical oxidation of p-aminophenol (PAP), following the enzymatic conversion of substrate-conjugated PAP. The calibration curves of β-galactosidase (β-gal), β-glucosidase (β-glu) and acid phosphatase (AcP) showed good linear correlation after being spiked in soils using chronoamperometry. -

Effective and Novel Application of Hydrodynamic Voltammetry to the Study of Superoxide Radical Scavenging by Natural Phenolic Antioxidants
antioxidants Article Effective and Novel Application of Hydrodynamic Voltammetry to the Study of Superoxide Radical Scavenging by Natural Phenolic Antioxidants Stuart Belli 1,*, Miriam Rossi 1,*, Nora Molasky 1, Lauren Middleton 1, Charles Caldwell 1, Casey Bartow-McKenney 1, Michelle Duong 1, Jana Chiu 1, Elizabeth Gibbs 1, Allison Caldwell 1, Christopher Gahn 2 and Francesco Caruso 1 1 Department of Chemistry, Vassar College, Poughkeepsie, NY 12604, USA; [email protected] (N.M.); [email protected] (L.M.); [email protected] (C.C.); [email protected] (C.B.-M.); [email protected] (M.D.); [email protected] (J.C.); [email protected] (E.G.); [email protected] (A.C.); [email protected] (F.C.) 2 Computing & Information Services, Vassar College, Poughkeepsie, NY 12604, USA; [email protected] * Correspondence: [email protected] (S.B.); [email protected] (M.R.) Received: 18 November 2018; Accepted: 25 December 2018; Published: 4 January 2019 Abstract: The reactions of antioxidants with superoxide radical were studied by cyclic voltammetry (CV)—and hydrodynamic voltammetry at a rotating ring-disk electrode (RRDE). In both methods, the superoxide is generated in solution from dissolved oxygen and then measured after being allowed to react with the antioxidant being studied. Both methods detected and measured the radical scavenging but the RRDE was able to give detailed insight into the antioxidant behavior. Three flavonoids, chrysin, quercetin and eriodictyol, were studied, their scavenging activity of superoxide was assessed and the molecular structure of each flavonoid was related to its scavenging capability. From our improved and novel RRDE method, we determine the ability of these 3 antioxidants to react with superoxide radical in a more quantitative manner than the classical CV. -

Metabotropic Glutamate Receptors
mGluR Metabotropic glutamate receptors mGluR (metabotropic glutamate receptor) is a type of glutamate receptor that are active through an indirect metabotropic process. They are members of thegroup C family of G-protein-coupled receptors, or GPCRs. Like all glutamate receptors, mGluRs bind with glutamate, an amino acid that functions as an excitatoryneurotransmitter. The mGluRs perform a variety of functions in the central and peripheral nervous systems: mGluRs are involved in learning, memory, anxiety, and the perception of pain. mGluRs are found in pre- and postsynaptic neurons in synapses of the hippocampus, cerebellum, and the cerebral cortex, as well as other parts of the brain and in peripheral tissues. Eight different types of mGluRs, labeled mGluR1 to mGluR8, are divided into groups I, II, and III. Receptor types are grouped based on receptor structure and physiological activity. www.MedChemExpress.com 1 mGluR Agonists, Antagonists, Inhibitors, Modulators & Activators (-)-Camphoric acid (1R,2S)-VU0155041 Cat. No.: HY-122808 Cat. No.: HY-14417A (-)-Camphoric acid is the less active enantiomer (1R,2S)-VU0155041, Cis regioisomer of VU0155041, is of Camphoric acid. Camphoric acid stimulates a partial mGluR4 agonist with an EC50 of 2.35 osteoblast differentiation and induces μM. glutamate receptor expression. Camphoric acid also significantly induced the activation of NF-κB and AP-1. Purity: ≥98.0% Purity: ≥98.0% Clinical Data: No Development Reported Clinical Data: No Development Reported Size: 10 mM × 1 mL, 100 mg Size: 10 mM × 1 mL, 5 mg, 10 mg, 25 mg (2R,4R)-APDC (R)-ADX-47273 Cat. No.: HY-102091 Cat. No.: HY-13058B (2R,4R)-APDC is a selective group II metabotropic (R)-ADX-47273 is a potent mGluR5 positive glutamate receptors (mGluRs) agonist. -

The Breakdown of Pyruvate by Cell-Free Extracts of the Rumen Micro-Organism LC
Vol. 74 METABOLISM OF XANTHURENIC ACID 525 with acid; no direct proof, however, is given that that the hydroxyl group in position 8 is bound to this transformation is brought about by detach- glucuronic acid. ment of a glucuronic residue from the hydroxyl group in position 4 of xanthurenic acid. The fact The authors are grateful to Professor Benassi of the that the R. values of Rothstein & Greenberg's Institute of Pharmaceutical Chemistry of the University of compounds B and D in butanol-acetic acid-water Padova for a kind gift of a sample of 8-methylxanthurenic (4:1:5) differ from those of our compounds indicate acid ethyl ester. that they are not the same substances. The differ- REFERENCES ence might tentatively be ascribed to the fact that xanthurenic acid can be conjugated in the living Baglioni, C., Fasella, P., Turano, C. & Siliprandi, N. (1960). organism in several ways and that differences exist G. Biochim. (in the Press). not only between various species, e.g. the rabbit Block, R. J. & Boiling, D. (1951). The Aminoacid Compo8i- and the rat (Rothstein & Greenberg, 1957), but tion of Protein and Foods, p. 413. Springfield, Mass.: also between different races of the same C. Thomas. species. Consden, R., Gordon, A. H. & Martin, A. J. P. (1944). Biochem. J. 38, 224. SUMMARY Dalglesh, C. E. (1952). Biochem. J. 52, 3. Dalgliesh, C. E. (1955). J. clin. Path. 8, 73. 1. Two major metabolites of xanthurenic acid Daigliesh, C. E. (1956). Biochem. J. 64, 481. were found in the urine of albino rats after intra- Dent, C. -

Modelling of the Rotating Disk Electrode in Ionic Liquids: Difference Between Water Based and Ionic Liquids Electrolytes
Modelling of the rotating disk electrode in Ionic liquids: difference between water based and ionic liquids electrolytes A. Giaccherini1, A. Lavacchi2 1INSTM, Firenze, Italy, 2ICCOM - CNR, Firenze, Italy *Corresponding author: via Lastruccia 3-13 50019, Sesto Fiorentino (FI), [email protected] Abstract: The last few years experienced a The former allows to validate the model by rapid growth in the application of Ionic means of comparison with the experimental Liquids (IL’s) to electrodeposition. ILs voltammograms, the last allows to offer a variety of advantages over aqueous rationalize the peculiar mass transport electrolytes. In general ILs show large properties of the Ils. In particular, thanks to chemical and thermal stability, high ionic the comparison of the concentration profiles conductivity and an electrochemical window and fluxes at the steady and quasi-steady much larger than water. These properties states of the potential scan for both systems, together with their negligible vapor pressure we clarified the nature of the unexpected enabling their use at different temperatures peaks show by the experimental without any risk of generating harmful voltammograms. vapors and joined to the absence of hydrogen discharge interfering with Keywords: Levich equation, Ionic Liquids, electrodeposition processes, as they are Transport proprieties, RDE, essentially hydrophobic, make them the best electroanalytical, CFD. candidates to be used for the obtainment of homogeneous electrodeposited thin films. 1. Introduction This study focuses on the silver electrodeposition from a silver Research on electrodeposition has recently tetrafluoroborate solution in 1-butyl-3- focused on the quest for new electrolytes methyltetrafluoroborate BMImBF4. We alternative to water. This was mainly driven notice that practical deposition rate at even by the need to develop new and green concentrations were much lower in ionic electrodeposition processes. -
Neurotransmitters-Sample-Report
Neurotransmitters Test the messengers of your neural network 1(866) 364-0963 www.vibrant-wellness.com 1360 Bayport Ave. Ste. B [email protected] San Carlos, CA 94070 Neurotransmitters Vibrant Wellness | 1360 Bayport Ave, Ste B. San Carlos, CA 94070 1(866) 364-0963 | [email protected] | www. vibrant-wellness.com Final Report Date: 06-19-2020 16:03 Specimen Collected: 06-18-2020 16:03 Accession ID: 2006190275 Specimen Received: 06-19-2020 10:03 LAST NAME FIRST NAME GENDER DATE OF BIRTH ACCESSION ID DATE OF SERVICE PATIENT TEST2 MALE 1998-01-06 2006190275 06-18-2020 16:03 PATIENT PROVIDER Name: TEST2 PATIENT Practice Name: Vibrant IT4 Practice Date of Birth: 1998-01-06 Provider Name: Demo Client, DDD (999994) Gender: Male Street Address: TEST STREET Age: 22 City: TEST CITY State: KY Fasting: FASTING Zip #: 42437 Telephone #: Fax #: 000-000-0000 Vibrant Wellness is pleased to present to you, 'Neurotransmitters', to help you make healthy lifestyle, dietary and treatment choices in consultation with your healthcare provider. It is intended to be used as a tool to encourage a general state of health and well-being. The Vibrant Neurotransmitters is a test to measure inhibitory, excitatory and other neurotransmitters . The panel is designed to give a complete picture of an individual’s levels of neurotransmitters in urine. Interpretation of Report: The report contains the complete list of the all urine neurotransmitters tested with quantitative results to enable a full overview along with the corresponding reference ranges. The classification of Red indicates a result that is outside the reference range and the classification of Green denotes a result that is within the reference range. -

Kynurenine and Tetrahydrobiopterin Pathways Crosstalk in Pain Hypersensitivity
fnins-14-00620 June 27, 2020 Time: 15:13 # 1 REVIEW published: 24 June 2020 doi: 10.3389/fnins.2020.00620 Kynurenine and Tetrahydrobiopterin Pathways Crosstalk in Pain Hypersensitivity Ananda Staats Pires1,2, Vanessa X. Tan1, Benjamin Heng1, Gilles J. Guillemin1* and Alexandra Latini2* 1 Neuroinflammation Group, Department of Biomedical Sciences, Centre for Motor Neuron Disease Research, Faculty of Medicine, Health and Human Sciences, Macquarie University, Sydney, NSW, Australia, 2 Laboratório de Bioenergética e Estresse Oxidativo, Departamento de Bioquímica, Centro de Ciências Biológicas, Universidade Federal de Santa Catarina, Florianópolis, Brazil Despite the identification of molecular mechanisms associated with pain persistence, no Edited by: significant therapeutic improvements have been made. Advances in the understanding Marianthi Papakosta, of the molecular mechanisms that induce pain hypersensitivity will allow the Takeda Pharmaceutical Company Limited, United States development of novel, effective, and safe therapies for chronic pain. Various pro- Reviewed by: inflammatory cytokines are known to be increased during chronic pain, leading Wladyslaw-Lason, to sustained inflammation in the peripheral and central nervous systems. The Institute of Pharmacology (PAS), Poland pro-inflammatory environment activates additional metabolic routes, including the Ewa Krystyna kynurenine (KYN) and tetrahydrobiopterin (BH4) pathways, which generate bioactive Szczepanska-Sadowska, soluble metabolites with the potential to modulate neuropathic and inflammatory pain Medical University of Warsaw, Poland sensitivity. Inflammation-induced upregulation of indoleamine 2,3-dioxygenase 1 (IDO1) *Correspondence: Gilles J. Guillemin and guanosine triphosphate cyclohydrolase I (GTPCH), both rate-limiting enzymes [email protected] of KYN and BH4 biosynthesis, respectively, have been identified in experimental Alexandra Latini [email protected] chronic pain models as well in biological samples from patients affected by chronic pain. -

Nutritional and Herbal Therapies for Children and Adolescents
Nutritional and Herbal Therapies for Children and Adolescents A Handbook for Mental Health Clinicians Nutritional and Herbal Therapies for Children and Adolescents A Handbook for Mental Health Clinicians George M. Kapalka Associate Professor, Monmouth University West Long Branch, NJ and Director, Center for Behavior Modifi cation Brick, NJ AMSTERDAM • BOSTON • HEIDELBERG • LONDON NEW YORK • OXFORD • PARIS • SAN DIEGO SAN FRANCISCO • SINGAPORE • SYDNEY • TOKYO Academic Press is an imprint of Elsevier Academic Press is an imprint of Elsevier 32 Jamestown Road, London NW1 7BY, UK 30 Corporate Drive, Suite 400, Burlington, MA 01803, USA 525 B Street, Suite 1900, San Diego, CA 92101-4495, USA Copyright © 2010 Elsevier Inc. All rights reserved No part of this publication may be reproduced, stored in a retrieval system or transmitted in any form or by any means electronic, mechanical, photocopying, recording or otherwise without the prior written permission of the publisher. Permissions may be sought directly from Elsevier’s Science & Technology Rights Department in Oxford, UK: phone (44) (0) 1865 843830; fax (44) (0) 1865 853333; email: [email protected]. Alternatively, visit the Science and Technology Books website at www.elsevierdirect.com/rights for further information Notice No responsibility is assumed by the publisher for any injury and/or damage to persons or property as a matter of products liability, negligence or otherwise, or from any use or operation of any methods, products, instructions or ideas contained in the material -

ALS Product Catalog Information
ALS Product Catalog Instrumentation Vol. 019A Working Electrodes Working Variety of products line up for research purposes Counter Electrodes RRDE-3A Reference ElectrodesReference Cells Voltammetry Flow Cells Model2325 SEC2020 Spectroelectrochemistry Others Electrochemistry General Catalog Information Technical notes and Movie library ALS technical notes and movie https://www.als-japan.com/technical-note.html frontpage --> Technical note ALS website has a "Technical note" and "Movie library" section, where you will find useful information and introduction movie of the products. For the instrument, set up and application movies will help you in the choose of the accessories. We will be always producing and releasing new movies, attending the demands of spectators. Inspection data sheet download service https://www.als-japan.com/dl/ Inspection data sheet link frontpage --> Support --> Electrode data ALS working and reference electrodes are tested and inspected before shipment, and the check data could be confirmed through the website. In the instruction manual, for the product which the check data is available, you will find the website direction. Product manual download service Instrumentation ALS Instruments instruction manual https://www.als-japan.com/support-instrument-manual.html Manual download link frontpage --> Support --> Instrument Manual Electrodes ALS support product manual https://www.als-japan.com/support-product-manual.html Manual download link frontpage --> Support --> Products Manual ALS product manual is available for download -

Bi Electrodeposition on Pt in Acidic Medium 2. Hydrodynamic Voltammetry
CHEMIJA. 2006. Vol. 17. No. 2–3. P. 11–15 © Lietuvos mokslų akademija,Bi electrodeposition 2006 on Pt in acidic medium. 2. Hydrodynamic voltammetry 11 © Lietuvos mokslų akademijos leidykla, 2006 Bi electrodeposition on Pt in acidic medium 2. Hydrodynamic voltammetry Ignas Valsiūnas*, A Pt rotating disc was used to provide some kinetic information concerning the electrodeposition of bismuth from Bi3+ acidic perchlorate solution 1 M Laima Gudavičiūtė, HClO4 + 0.05 M Bi(ClO4)3 at 20 °C. The electrodeposition of Bi from this solution may be interpreted in terms of an irreversible stepwise discharge of Vidmantas Kapočius and Bi3+ ions proceeding either through three successive one-electron steps with the transfer of the first electron as the rate-limiting step or via two successive Antanas Steponavičius steps with the transfer of two electrons in the first stage as the rate-limiting step. From the experimental data the diffusion coefficient D for Bi3+ ion was Institute of Chemistry, calculated and was found to be 4.9 · 10-6 cm2 s-1. A. Goštauto 9, LT-01108 Vilnius, Lithuania Key words: bismuth, electrodeposition, perchlorate solutions, Pt rotating disc electrode, rate-determining steps INTRODUCTION technique was therefore employed in order to elucidate the further information on the mechanism of Bi Although bismuth, as a semimetal, exhibits some unusual electrochemical deposition on a polycrystalline Pt thermal, electrical and magnetic properties that make its (Pt(poly)) electrode. actual and potential applications [1, 2] to be rather wide, its electrochemical reduction from Bi3+ solutions has not EXPERIMENTAL been studied very extensively. Several electrochemical studies have been devoted to the investigation of current- Details on the preparation of perchlorate Bi3+ solution, potential (i/E) characteristics [3–7]. -
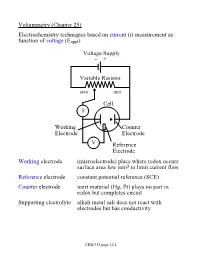
Voltammetry (Chapter 25) Electrochemistry Techniques Based on Current (I) Measurement As Function of Voltage (Eappl)
Voltammetry (Chapter 25) Electrochemistry techniques based on current (i) measurement as function of voltage (Eappl) Voltage Supply - + Variable Resistor max min Cell I Working Counter Electrode Electrode V Reference Electrode Working electrode (microelectrode) place where redox occurs surface area few mm2 to limit current flow Reference electrode constant potential reference (SCE) Counter electrode inert material (Hg, Pt) plays no part in redox but completes circuit Supporting electrolyte alkali metal salt does not react with electrodes but has conductivity CEM 333 page 12.1 Why not use 2 electrodes? OK in potentiometry - very small currents. Now, want to measure current (larger=better) but • potential drops when current is taken from electrode (IR drop) • must minimize current withdrawn from reference electrode surface Potentiostat (voltage source) drives cell • supplies whatever voltage needed between working and counter electrodes to maintain specific voltage between working and reference electrode NOTE: • Almost all current carried between working and counter electrodes • Voltage measured between working and reference electrodes • Analyte dissolved in cell not at electrode surface! CEM 333 page 12.2 Excitation signals (Fig 25-2) CEM 333 page 12.3 Microelectrodes C, Au, Pt, Hg each useful in certain solutions/voltage ranges Fig 25-4 At -ve limit, oxidation of water + - 2H2O ® 4H + O2(g) + 4e At +ve limit, reduction of water - - 2H2O + 2e ® H2 + 2OH CEM 333 page 12.4 Varies with material/solution due to different overpotentials Overpotential