Ankle and Foot Disorders Guideline
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Volume 15, Issue 1, January-April
Volume 15, Issue 1, January-April Osteochondral lesions of the talus in adults J. Batista, G. Joannas, L. Casola, L. Logioco, G. Arrondo 1A Traumatic lesion with isolated cartilage injury (flap) Tx: arthroscopy, curettage, and microfractures. 1B Traumatic lesion (cartilage and subchondral bone injury) 1B.1 Lesion <10mm in diameter and <5mm of depth (superficial lesion) Tx: arthroscopy, curettage, and microfractures. 1B.2 Lesion >10mm in diameter and >5mm in depth Tx: fragment fixation with osteosynthesis, open surgery, osteochondral graft, or mosaicoplasty. 2A Non-traumatic isolated bone injury, subchondral cyst. Tx: retrograde drilling. 2B Non-traumatic open subchondral bone cyst with articular connection (progression of type 2A). 2B.1 Lesion measuring <10mm in diameter and <5mm in depth (superficial lesion). Tx: arthroscopy, curettage, and microfractures. 2B.2 Lesion measuring >10mm in diameter and >5mm in depth. Tx: open surgery, osteochondral graft, or mosaicoplasty. 3 Type 1 or 2 lesions associated with lateral instability of the ankle Tx: ligament repair. 4 With limb deformities 4A Types 1 or 2 lesions with hindfoot deformity = varus or valgus calcaneus Tx: varus or valgus calcaneal osteotomy. 4B Type 1 or 2 lesion with supramalleolar deformity of distal tibia (varus or valgus) Tx: varus or valgus supramalleolar osteotomy. Tx: treatment. Volume 15, Issue 1, January-April The Journal of the Foot & Ankle (eISSN 2675-2980) is published quarterly in April, August, and December, with the purpose of disseminating papers on themes of Foot and Ankle Medicine and Surgery and related areas. The Journal offers free and open access to your content on our website. All papers are already published with active DOIs. -

Anterior Reconstruction Techniques for Cervical Spine Deformity
Neurospine 2020;17(3):534-542. Neurospine https://doi.org/10.14245/ns.2040380.190 pISSN 2586-6583 eISSN 2586-6591 Review Article Anterior Reconstruction Techniques Corresponding Author for Cervical Spine Deformity Samuel K. Cho 1,2 1 1 1 https://orcid.org/0000-0001-7511-2486 Murray Echt , Christopher Mikhail , Steven J. Girdler , Samuel K. Cho 1Department of Orthopedics, Icahn School of Medicine at Mount Sinai, New York, NY, USA Department of Orthopaedics, Icahn 2 Department of Neurological Surgery, Montefiore Medical Center/Albert Einstein College of Medicine, Bronx, School of Medicine at Mount Sinai, 425 NY, USA West 59th Street, 5th Floor, New York, NY, USA E-mail: [email protected] Cervical spine deformity is an uncommon yet severely debilitating condition marked by its heterogeneity. Anterior reconstruction techniques represent a familiar approach with a range Received: June 24, 2020 of invasiveness and correction potential—including global or focal realignment in the sagit- Revised: August 5, 2020 tal and coronal planes. Meticulous preoperative planning is required to improve or prevent Accepted: August 17, 2020 neurologic deterioration and obtain satisfactory global spinal harmony. The ability to per- form anterior only reconstruction requires mobility of the opposite column to achieve cor- rection, unless a combined approach is planned. Anterior cervical discectomy and fusion has limited focal correction, but when applied over multiple levels there is a cumulative ef- fect with a correction of approximately 6° per level. Partial or complete corpectomy has the ability to correct sagittal deformity as well as decompress the spinal canal when there is an- terior compression behind the vertebral body. -

Association of Generalized Joint Hypermobility and the Occurrence of Musculoskeletal Work-Related Injury in the First Zero to Fi
University of North Dakota UND Scholarly Commons Physical Therapy Scholarly Projects Department of Physical Therapy 2018 Association of Generalized Joint Hypermobility and the Occurrence of Musculoskeletal Work- Related Injury in the First Zero to Five Years of Physical Therapy Practice: A Pilot Study Mikelle Fetsch University of North Dakota Ashley Naas University of North Dakota Amanda Slaikeu University of North Dakota Follow this and additional works at: https://commons.und.edu/pt-grad Part of the Physical Therapy Commons Recommended Citation Fetsch, Mikelle; Naas, Ashley; and Slaikeu, Amanda, "Association of Generalized Joint Hypermobility and the Occurrence of Musculoskeletal Work-Related Injury in the First Zero to Five Years of Physical Therapy Practice: A Pilot Study" (2018). Physical Therapy Scholarly Projects. 655. https://commons.und.edu/pt-grad/655 This Scholarly Project is brought to you for free and open access by the Department of Physical Therapy at UND Scholarly Commons. It has been accepted for inclusion in Physical Therapy Scholarly Projects by an authorized administrator of UND Scholarly Commons. For more information, please contact [email protected]. ASSOCIATION OF GENERALIZED JOINT HYPERMOBlLlTY AND THE OCCURRENCE OF MUSCULOSKELETAL WORK-RELATED INJURY IN THE FIRST ZERO TO FIVE YEARS OF PHYSICAL THERAPY PRACTICE: A PILOT STUDY by Mikelle Fetsch Bachelor of General Stndies with a Health Sciences Emphasis University of North Dakota, 2016 Ashley Naas Bachelor of General Studies with a Health Sciences Emphasis -

1. MOA AAA 2016 Abstract
Abstract Combined Meeting of the th Malaysian Orthopaedic 46Association Annual General Meeting / Annual Scientific Meeting th ASEAN Arthroplasty 10 Association Meeting 2016 Fundamentals In Orthopaedics – Back To Basics Pre-Conference Day Conference Days 25th May 2016 26th to 28th May 2016 Persada Johor International Convention Centre, Johor Bahru, Malaysia. www.moa-home.com Abstract CD (Please click on the links below to view the respective categories of abstracts.) Oral Presentations Abstracts Poster Presentations Abstracts (Click Here...) Combined Meeting of the 46th Malaysian Orthopaedic Association Annual General Meeting / Annual Scientific Meeting & 10th ASEAN Arthroplasty Association Meeting 2016 26th May 2016 (Thursday) - Lecture Hall MOA 1, Level 3 TIME TOPIC SPEAKER 0700 -1730 REGISTRATION COUNTER OPENS SUBIR SENGUPTA MEMORIAL LECTURE Chairperson Prof Dr Saw Aik 0830 - 0900 Prevention And Early Detection Of DDH - The Japanese SM 01 Prof Dr Makoto Kamegaya Experience OPENING CEREMONY 0900 - 1030 Orthopaedics At The Frontlines In A Changing Globalised World. SK 01 Roles And Responsibilities. Dato' Dr Ahmad Faizal Mohd Perdaus A View From A Humanitarian And Colleauge. 1030 - 1100 TEA BREAK & EXHIBIT VISIT SPORTS Dr Shamsul Iskandar Hussein Chairperson Dr Raymond Yeak Dieu Kiat Revision Anterior Cruciate Ligament Reconstruction: Analysis 1100 - 1112 SX 01 Of Causes Of Failures, Preoperative Clinical Evaluation And Dr Deepak V. Patel Planning, Surgical Technique, And Clinical Outcomes SLAP (Superior Labrum Anterior Posterior) -

Sports Medicine Examination Outline
Sports Medicine Examination Content I. ROLE OF THE TEAM PHYSICIAN 1% A. Ethics B. Medical-Legal 1. Physician responsibility 2. Physician liability 3. Preparticipation clearance 4. Return to play 5. Waiver of liability C. Administrative Responsibilities II. BASIC SCIENCE OF SPORTS 16% A. Exercise Physiology 1. Training Response/Physical Conditioning a.Aerobic b. Anaerobic c. Resistance d. Flexibility 2. Environmental a. Heat b.Cold c. Altitude d.Recreational diving (scuba) 3. Muscle a. Contraction b. Lactate kinetics c. Delayed onset muscle soreness d. Fiber types 4. Neuroendocrine 5. Respiratory 6. Circulatory 7. Special populations a. Children b. Elderly c. Athletes with chronic disease d. Disabled athletes B. Anatomy 1. Head/Neck a.Bone b. Soft tissue c. Innervation d. Vascular 2. Chest/Abdomen a.Bone b. Soft tissue c. Innervation d. Vascular 3. Back a.Bone b. Soft tissue c. Innervation 1 d. Vascular 4. Shoulder/Upper arm a. Bone b. Soft tissue c. Innervation d. Vascular 5. Elbow/Forearm a. Bone b. Soft tissue c. Innervation d. Vascular 6. Hand/Wrist a. Bone b. Soft tissue c. Innervation d. Vascular 7. Hip/Pelvis/Thigh a. Bone b. Soft tissue c. Innervation d. Vascular 8. Knee a. Bone b. Soft tissue c. Innervation d. Vascular 9. Lower Leg/Foot/Ankle a. Bone b. Soft tissue c. Innervation d. Vascular 10. Immature Skeleton a. Physes b. Apophyses C. Biomechanics 1. Throwing/Overhead activities 2. Swimming 3. Gait/Running 4. Cycling 5. Jumping activities 6. Joint kinematics D. Pharmacology 1. Therapeutic Drugs a. Analgesics b. Antibiotics c. Antidiabetic agents d. Antihypertensives e. -

SPA Referral Guidelines
SUTTER PHYSICIANS ALLIANCE (SPA) 2800 L Street, 7th Floor Sacramento, CA 95816 SPA Specialty Referral Guideline Pittsburg Knee Rules for Ordering Radiology Ottawa Ankle Rules for Ordering Radiology Developed May 10, 2005 Revised April 23, 2007 Reviewed July 27, 2009 I. Indications for Ordering X-ray of the Knee.......................................Page 2 II. Indications for Ordering X-ray of the Ankle .....................................Page 2 III. Indications for Ordering X-ray of the Foot ........................................Page 2 IV. Exclusion Criterion for Ankle and Foot.............................................Page 2 SPA Specialty Referral Guideline – Pittsburg Knee / Ottawa Ankle Referral Indications Revised 4/23/07 Page 2 of 2 I. Indications for Ordering X-ray of the Knee Pittsburg Knee Rules/Indications for Ordering Plain Films of the Knee A) If the patient experienced a fall or blunt trauma, and is unable to walk four (4) weight- bearing steps, an X-ray is indicated. B) If the patient experienced a fall or blunt trauma, and is under 12 or over 50 years of age, an X-ray is indicated. If the above criteria are not present, no need for X-ray. II. Indications for Ordering X-ray of the Ankle Ottawa Ankle Rules Pain in the malleolar zone and ANY of the following: A) Bony tenderness at posterior edge of distal 6cm of the lateral malleolus. B) Bony tenderness at posterior edge of distal 6cm of the medial malleolus. C) Inability to weight-bear immediately. III. Indications for Ordering X-ray of the Foot Pain in the mid-foot zone and ANY of the following: A) Bony tenderness at the base of the 5th metatarsal. -

Surgical Treatment of Traumatic Cervical Facet Dislocation
DOI: 10.1590/0004-282X20160078 VIEW AND REVIEW Surgical treatment of traumatic cervical facet dislocation: anterior, posterior or combined approaches? Deslocamentos facetários cervicais traumáticos: abordagem anterior, posterior ou combinada? Catarina C. Lins1, Diego T. Prado2, Andrei F. Joaquim1,3 ABSTRACT Surgical treatment is well accepted for patients with traumatic cervical facet joint dislocations (CFD), but there is uncertainty over which approach is better: anterior, posterior or combined. We performed a systematic literature review to evaluate the indications for anterior and posterior approaches in the management of CFD. Anterior approaches can restore cervical lordosis, and cause less postoperative pain and less wound problems. Posterior approaches are useful for direct reduction of locked facet joints and provide stronger fixation from a biomechanical point of view. Combined approaches can be used in more complex cases. Although both anterior and posterior approaches can be used interchangeably, there are some patients who may benefit from one of them over the other, as discussed in this review. Surgeons who treat cervical spine trauma should be able to perform both procedures as well as combined approaches to adequately manage CFD and improve patients’ final outcomes. Keywords: spine; dislocations; bones fractures; surgery. RESUMO O tratamento dos deslocamentos facetários cervicais traumáticos (DFC) é preferencialmente cirúrgico, conforme a literatura pertinente, mas há dúvidas quanto a melhor forma de abordagem da coluna: anterior, posterior ou combinada. Realizamos revisão sistemática para avaliar as indicações da abordagem anterior e da posterior nos DFC. A abordagem anterior permite restaurar a lordose cervical, com menor dor no pós-operatório e menos problemas relacionados a ferida cirúrgica. -

A Case of Bilateral Calcaneal Fracture, Following Fall from 35 Feet
rauma & Sastry et al., J Trauma Treat 2015, 4:3 f T T o re l a t a m DOI: 10.4172/2167-1222.1000254 n r e u n o t J Journal of Trauma & Treatment ISSN:ISSN: 2167-1222 2167-1222 CaseResearch Report Article OpenOpen Access Access A Case of Bilateral Calcaneal Fracture, Following Fall from 35 Feet Purushothama Sastry, Sujana Theja JS, Supreeth N and Arjun Markanday* Department of Orthopaedics, JSS Hospital, Mysore, India Abstract Introduction: Called Lover’s Fractures, the calcaneus commonly fractures due to fall from height. The calcaneus is the most frequently fractured tarsal bone. Tarsal bone fractures account for about 2% of all adult fractures. Of these, 60% are calcaneus fractures.The heel bone is often injured in a high-energy collision where other parts of the skeleton are also injured. In up to 10% of cases, the patient can also sustain a fracture of the spine, hip, or the other calcaneus. Injuries to the calcaneus often damage the subtalar joint and cause the joint to become stiff. This makes it difficult to walk on uneven ground or slanted surfaces. Case Report: A 24 year old male, working as a lift operator presented to the casualty of the hospital after the elevator broke down and came down in a free fall from about a height of 4 stories (35 feet). He presented along with the other occupants of the lift who also sustained calcaneal fractures.The case on arrival was subjected to ATLS protocol and through radiologic work up was done. Following a period of 12 days post trauma he was operated for bilateral calcaneal fractures and discharged 10 days post operatively. -

S41598-020-78754-9.Pdf
www.nature.com/scientificreports OPEN Acromioclavicular and sternoclavicular joint dislocations indicate severe concomitant thoracic and upper extremity injuries in severely injured patients M. Sinan Bakir1,2*, Rolf Lefering3, Lyubomir Haralambiev1,2, Simon Kim1, Axel Ekkernkamp1,2, Denis Gümbel1,2 & Stefan Schulz‑Drost2,4,5 Preliminary studies show that clavicle fractures (CF) are known as an indicator in the severely injured for overall injury severity that are associated with relevant concomitant injuries in the thorax and upper extremity. In this regard, little data is available for the rarer injuries of the sternoclavicular and acromioclavicular joints (SCJ and ACJ, respectively). Our study will answer whether clavicular joint injuries (CJI), by analogy, have a similar relevance for the severely injured. We performed an analysis from the TraumaRegister DGU (TR‑DGU). The inclusion criterion was an Injury Severity Score (ISS) of at least 16. In the TR‑DGU, the CJI were registered as one entity. The CJI group was compared with the CF and control groups (those without any clavicular injuries). Concomitant injuries were distinguished using the Abbreviated Injury Scale according to their severity. The inclusion criteria were met by n = 114,595 patients. In the case of CJI, n = 1228 patients (1.1%) were found to be less severely injured than the controls in terms of overall injury severity. Compared to the CF group (n = 12,030; 10.5%) with higher ISS than the controls, CJI cannot be assumed as an indicator for a more severe trauma; however, CF can. Concomitant injuries were more common for severe thoracic and moderate upper extremity injuries than other body parts for CJI. -
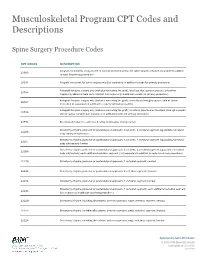
Musculoskeletal Program CPT Codes and Descriptions
Musculoskeletal Program CPT Codes and Descriptions Spine Surgery Procedure Codes CPT CODES DESCRIPTION Allograft, morselized, or placement of osteopromotive material, for spine surgery only (List separately in addition 20930 to code for primary procedure) 20931 Allograft, structural, for spine surgery only (List separately in addition to code for primary procedure) Autograft for spine surgery only (includes harvesting the graft); local (eg, ribs, spinous process, or laminar 20936 fragments) obtained from same incision (List separately in addition to code for primary procedure) Autograft for spine surgery only (includes harvesting the graft); morselized (through separate skin or fascial 20937 incision) (List separately in addition to code for primary procedure) Autograft for spine surgery only (includes harvesting the graft); structural, bicortical or tricortical (through separate 20938 skin or fascial incision) (List separately in addition to code for primary procedure) 20974 Electrical stimulation to aid bone healing; noninvasive (nonoperative) Osteotomy of spine, posterior or posterolateral approach, 3 columns, 1 vertebral segment (eg, pedicle/vertebral 22206 body subtraction); thoracic Osteotomy of spine, posterior or posterolateral approach, 3 columns, 1 vertebral segment (eg, pedicle/vertebral 22207 body subtraction); lumbar Osteotomy of spine, posterior or posterolateral approach, 3 columns, 1 vertebral segment (eg, pedicle/vertebral 22208 body subtraction); each additional vertebral segment (List separately in addition to code for -

The Ehlers–Danlos Syndromes
PRIMER The Ehlers–Danlos syndromes Fransiska Malfait1 ✉ , Marco Castori2, Clair A. Francomano3, Cecilia Giunta4, Tomoki Kosho5 and Peter H. Byers6 Abstract | The Ehlers–Danlos syndromes (EDS) are a heterogeneous group of hereditary disorders of connective tissue, with common features including joint hypermobility, soft and hyperextensible skin, abnormal wound healing and easy bruising. Fourteen different types of EDS are recognized, of which the molecular cause is known for 13 types. These types are caused by variants in 20 different genes, the majority of which encode the fibrillar collagen types I, III and V, modifying or processing enzymes for those proteins, and enzymes that can modify glycosaminoglycan chains of proteoglycans. For the hypermobile type of EDS, the molecular underpinnings remain unknown. As connective tissue is ubiquitously distributed throughout the body, manifestations of the different types of EDS are present, to varying degrees, in virtually every organ system. This can make these disorders particularly challenging to diagnose and manage. Management consists of a care team responsible for surveillance of major and organ-specific complications (for example, arterial aneurysm and dissection), integrated physical medicine and rehabilitation. No specific medical or genetic therapies are available for any type of EDS. The Ehlers–Danlos syndromes (EDS) comprise a genet six EDS types, denominated by a descriptive name6. The ically heterogeneous group of heritable conditions that most recent classification, the revised EDS classification in share several clinical features, such as soft and hyper 2017 (Table 1) identified 13 distinct clinical EDS types that extensible skin, abnormal wound healing, easy bruising are caused by alterations in 19 genes7. -

EM Cases Digest Vol. 1 MSK & Trauma
THE MAGAZINE SERIES FOR ENHANCED EM LEARNING Vol. 1: MSK & Trauma Copyright © 2015 by Medicine Cases Emergency Medicine Cases by Medicine Cases is copyrighted as “All Rights Reserved”. This eBook is Creative Commons Attribution-NonCommercial- NoDerivatives 3.0 Unsupported License. Upon written request, however, we may be able to share our content with you for free in exchange for analytic data. For permission requests, write to the publisher, addressed “Attention: Permissions Coordinator,” at the address below. Medicine Cases 216 Balmoral Ave Toronto, ON, M4V 1J9 www.emergencymedicinecases.com This book has been authored with care to reflect generally accepted practices. As medicine is a rapidly changing field, new diagnostic and treatment modalities are likely to arise. It is the responsibility of the treating physician, relying on his/her experience and the knowledge of the patient, to determine the best management plan for each patient. The author(s) and publisher of this book are not responsible for errors or omissions or for any consequences from the application of the information in this book and disclaim any liability in connection with the use of this information. This book makes no guarantee with respect to the completeness or accuracy of the contents within. OUR THANKS TO... EDITORS IN CHIEF Anton Helman Taryn Lloyd PRODUCTION EDITOR Michelle Yee PRODUCTION MANAGER Garron Helman CHAPTER EDITORS Niran Argintaru Michael Misch PODCAST SUMMARY EDITORS Lucas Chartier Keerat Grewal Claire Heslop Michael Kilian PODCAST GUEST EXPERTS Andrew Arcand Natalie Mamen Brian Steinhart Mike Brzozowski Hossein Mehdian Arun Sayal Ivy Cheng Sanjay Mehta Laura Tate Walter Himmel Jonathan Pirie Rahim Valani Dave MacKinnon Jennifer Riley University of Toronto, Faculty of Medicine EM Cases is a venture of the Schwartz/ Reisman Emergency Medicine Institute.