Dienogest/Ethinylestradiol: Slightly Higher Relative Risk of Venous Thromboembolism
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ocps) Used in the NM Family Planning Program (FPP
The following slides are intended to familiarize nurses and clinicians with oral contraceptive pills (OCPs) used in the NM Family Planning Program (FPP). The FPP does not intend for this information to supersede the Family Planning Protocol particularly on the requirement for Public Health Nurses in the Public Health Offices to consult a clinician as stated in the Protocol when in doubt or if it is necessary to switch the client’s OCP type. 1 2 Combined oral contraceptives (COCs) contain two hormones; estrogen and progestin. In general, any combined OCP is good for most women who are eligible to take estrogen according to the CDC U.S. Medical Eligibility Criteria (MEC). Once again, refer to the US MEC chart to find out if OCP is a suitable choice for clients with specific health conditions. To learn a little bit about what each hormone does, the FPP is providing the following summary: Estrogen: provides endometrial stability = menstrual cycle control. A higher estrogen dose increases the venous thromboembolism (VTE) or clot risk but OCP clot risk is still less harmful than the clot risk related to pregnancy and giving birth. Progestin: provides most of the contraceptive effect by ‐Preventing luteinizing hormone (LH) surge /ovulation ‐Thickening the cervical mucus to prevent sperm entry. Two major OCP formations are available. Monophasic: There is only one dose of estrogen and progestin in each active pill in the packet; and Multiphasic: There are varying doses of hormones, particularly progestin in the active pills. 3 Section 3 of the FPP Protocol contains the OCP Substitute Table, which groups OCPs into 6 classes according to the estrogen dosage, the type of progestin and the formulations. -

Download PDF File
Ginekologia Polska 2019, vol. 90, no. 9, 520–526 Copyright © 2019 Via Medica ORIGINAL PAPER / GYNECologY ISSN 0017–0011 DOI: 10.5603/GP.2019.0091 Anti-androgenic therapy in young patients and its impact on intensity of hirsutism, acne, menstrual pain intensity and sexuality — a preliminary study Anna Fuchs, Aleksandra Matonog, Paulina Sieradzka, Joanna Pilarska, Aleksandra Hauzer, Iwona Czech, Agnieszka Drosdzol-Cop Department of Pregnancy Pathology, Department of Woman’s Health, School of Health Sciences in Katowice, Medical University of Silesia, Katowice, Poland ABSTRACT Objectives: Using anti-androgenic contraception is one of the methods of birth control. It also has a significant, non-con- traceptive impact on women’s body. These drugs can be used in various endocrinological disorders, because of their ability to reduce the level of male hormones. The aim of our study is to establish a correlation between taking different types of anti-androgenic drugs and intensity of hirsutism, acne, menstrual pain intensity and sexuality . Material and methods: 570 women in childbearing age that had been using oral contraception for at least three months took part in our research. We examined women and asked them about quality of life, health, direct causes and effects of that treatment, intensity of acne and menstrual pain before and after. Our research group has been divided according to the type of gestagen contained in the contraceptive pill: dienogest, cyproterone, chlormadynone and drospirenone. Ad- ditionally, the control group consisted of women taking oral contraceptives without antiandrogenic component. Results: The mean age of the studied group was 23 years ± 3.23. 225 of 570 women complained of hirsutism. -

Comparing the Effects of Combined Oral Contraceptives Containing Progestins with Low Androgenic and Antiandrogenic Activities on the Hypothalamic-Pituitary-Gonadal Axis In
JMIR RESEARCH PROTOCOLS Amiri et al Review Comparing the Effects of Combined Oral Contraceptives Containing Progestins With Low Androgenic and Antiandrogenic Activities on the Hypothalamic-Pituitary-Gonadal Axis in Patients With Polycystic Ovary Syndrome: Systematic Review and Meta-Analysis Mina Amiri1,2, PhD, Postdoc; Fahimeh Ramezani Tehrani2, MD; Fatemeh Nahidi3, PhD; Ali Kabir4, MD, MPH, PhD; Fereidoun Azizi5, MD 1Students Research Committee, School of Nursing and Midwifery, Department of Midwifery and Reproductive Health, Shahid Beheshti University of Medical Sciences, Tehran, Islamic Republic Of Iran 2Reproductive Endocrinology Research Center, Research Institute for Endocrine Sciences, Shahid Beheshti University of Medical Sciences, Tehran, Islamic Republic Of Iran 3School of Nursing and Midwifery, Department of Midwifery and Reproductive Health, Shahid Beheshti University of Medical Sciences, Tehran, Islamic Republic Of Iran 4Minimally Invasive Surgery Research Center, Iran University of Medical Sciences, Tehran, Islamic Republic Of Iran 5Endocrine Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Islamic Republic Of Iran Corresponding Author: Fahimeh Ramezani Tehrani, MD Reproductive Endocrinology Research Center Research Institute for Endocrine Sciences Shahid Beheshti University of Medical Sciences 24 Parvaneh Yaman Street, Velenjak, PO Box 19395-4763 Tehran, 1985717413 Islamic Republic Of Iran Phone: 98 21 22432500 Email: [email protected] Abstract Background: Different products of combined oral contraceptives (COCs) can improve clinical and biochemical findings in patients with polycystic ovary syndrome (PCOS) through suppression of the hypothalamic-pituitary-gonadal (HPG) axis. Objective: This systematic review and meta-analysis aimed to compare the effects of COCs containing progestins with low androgenic and antiandrogenic activities on the HPG axis in patients with PCOS. -

PMBJP Product.Pdf
Sr. Drug Generic Name of the Medicine Unit Size MRP Therapeutic Category No. Code Analgesic & Antipyretic / Muscle 1 1 Aceclofenac 100mg and Paracetamol 325 mg Tablet 10's 10's 8.00 relaxants Analgesic & Antipyretic / Muscle 2 2 Aceclofenac Tablets IP 100mg 10's 10's 4.37 relaxants Acetaminophen 325 + Tramadol Hydrochloride 37.5 film Analgesic & Antipyretic / Muscle 3 4 10's 8.00 coated Tablet 10's relaxants Analgesic & Antipyretic / Muscle 4 5 ASPIRIN Tablets IP 150 mg 14's 14's 2.70 relaxants DICLOFENAC 50 mg+ PARACETAMOL 325 mg+ Analgesic & Antipyretic / Muscle 5 6 10's 11.30 CHLORZOXAZONE 500 mg Tablets 10's relaxants Diclofenac Sodium 50mg + Serratiopeptidase 10mg Tablet Analgesic & Antipyretic / Muscle 6 8 10's 12.00 10's relaxants Analgesic & Antipyretic / Muscle 7 9 Diclofenac Sodium (SR) 100 mg Tablet 10's 10's 6.12 relaxants Analgesic & Antipyretic / Muscle 8 10 Diclofenac Sodium 25mg per ml Inj. IP 3 ml 3 ml 2.00 relaxants Analgesic & Antipyretic / Muscle 9 11 Diclofenac Sodium 50 mg Tablet 10's 10's 2.90 relaxants Analgesic & Antipyretic / Muscle 10 12 Etoricoxilb Tablets IP 120mg 10's 10's 33.00 relaxants Analgesic & Antipyretic / Muscle 11 13 Etoricoxilb Tablets IP 90mg 10's 10's 25.00 relaxants Analgesic & Antipyretic / Muscle 12 14 Ibuprofen 400 mg + Paracetamol 325 mg Tablet 10's 15's 5.50 relaxants Analgesic & Antipyretic / Muscle 13 15 Ibuprofen 200 mg film coated Tablet 10's 10's 1.80 relaxants Analgesic & Antipyretic / Muscle 14 16 Ibuprofen 400 mg film coated Tablet 10's 15's 3.50 relaxants Analgesic & Antipyretic -

ORTHO TRI-CYCLEN® TABLETS ORTHO-CYCLEN® TABLETS (Norgestimate/Ethinyl Estradiol)
PHYSICIANS' PACKAGE INSERT ORTHO TRI-CYCLEN® TABLETS ORTHO-CYCLEN® TABLETS (norgestimate/ethinyl estradiol) Patients should be counseled that this product does not protect against HIV infection (AIDS) and other sexually transmitted diseases. DESCRIPTION Each of the following products is a combination oral contraceptive containing the progestational compound norgestimate and the estrogenic compound ethinyl estradiol. ORTHO TRI-CYCLEN 21 Tablets and ORTHO TRI-CYCLEN 28 Tablets. Each white tablet contains 0.180 mg of the progestational compound, norgestimate (18,19-Dinor- 17-pregn-4-en-20-yn-3-one,17-(acetyloxy)-13-ethyl-, oxime,(17α)-(+)-) and 0.035 mg of the estrogenic compound, ethinyl estradiol (19-nor-17α-pregna,1,3,5(10)-trien-20-yne-3,17-diol). Inactive ingredients include lactose, magnesium stearate, and pregelatinized starch. Each light blue tablet contains 0.215 mg of the progestational compound norgestimate (18,19- Dinor-17-pregn-4-en-20-yn-3-one,17-(acetyloxy)-13-ethyl-,oxime,(17α)-(+)-) and 0.035 mg of the estrogenic compound, ethinyl estradiol (19-nor-17α-pregna,1,3,5(10)-trien-20-yne-3,17-diol). Inactive ingredients include FD & C Blue No. 2 Aluminum Lake, lactose, magnesium stearate, and pregelatinized starch. Each blue tablet contains 0.250 mg of the progestational compound norgestimate (18,19-Dinor-17- pregn-4-en-20-yn-3-one, 17-(acetyloxy)-13-ethyl-,oxime,(17α)-(+)-) and 0.035 mg of the estrogenic compound, ethinyl estradiol (19-nor-17α-pregna,1,3,5(10)-trien-20-yne-3,17-diol). Inactive ingredients include FD & C Blue No. 2 Aluminum Lake, lactose, magnesium stearate, and pregelatinized starch. -

Desogestrel-Only Pill (Cerazette)
J Fam Plann Reprod Health Care: first published as 10.1783/147118903101197593 on 1 July 2003. Downloaded from Faculty of Family Planning and Reproductive Health Care Clinical Effectiveness Unit A unit funded by the FFPRHC and supported by the University of Aberdeen and SPCERH to provide guidance on evidence-based practice New Product Review (April 2003) Desogestrel-only Pill (Cerazette) Journal of Family Planning and Reproductive Health Care 2003; 29(3): 162–164 Evidence from a randomised trial has shown that a 75 mg (microgrammes) desogestrel pill inhibits ovulation in 97% of cycles. Thus, on theoretical grounds, we would expect the desogestrel pill to be more effective than existing progestogen- only pills (POPs). However, Pearl indices from clinical trials comparing it to a levonorgestrel POP were not significantly different. Therefore an evidence-based recommendation cannot be made that the desogestrel pill is different from other POPs in terms of efficacy, nor that it is similar to combined oral contraception (COC) in this respect. An evidence-based recommendation can be made that the desogestrel-only pill is similar to other POPs in terms of side effects and acceptability. The desogestrel-only pill is not recommended as an alternative to COC in routine practice, but provides a useful alternative for women who require oestrogen-free contraception. In clinical trials: l Ovulation was inhibited in 97% of cycles at 7 and 12 months after initiation. l The Pearl index was 0.41 per 100 woman-years, which was not significantly different from a levonorgestrel-only pill. However, the trial providing these data was too small to detect a clinically important difference. -

Estrogen and Progestin Hormone Doses in Combined Birth Control Pills
Estrogen and Progestin Hormone Doses in Combined Birth Control Pills Estrogen level Pill Brand Name Progestin Dose (mg) ethinyl estradiol (micrograms) 20 mcgm Alesse® levonorgestrel 0.10 Levlite® levonorgestrel 0.10 Loestrin 1/20® Fe norethindrone 1.00 acetate Mircette® desogestrel 0.15 Ortho Evra® norelgestromin 0.15 (patch) (norgestimate metabolite) phasic Estrostep® Fe norethindrone 1.0/1.0/1.0 20/30/35 mcgm acetate 30 mcgm Levlen® levonorgestrel 0.15 Levora® levonorgestrel 0.15 Nordette® levonorgestrel 0.15 Lo/Ovral® norgestrel 0.30 Desogen® desogestrel 0.15 Ortho-Cept® desogestrel 0.15 Loestrin® 1.5/30 norethindrone 1.50 acetate Yasmin® drospirenone 3.0 phasic Triphasil® levonorgestrel 0.05/0.075/0.125 30/40/30 mcgm Tri-Levlen® levonorgestrel 0.05/0.075/0.125 Trivora® levonorgestrel 0.05/0.075/0.125 35 mcgm Ortho-Cyclen® norgestimate 0.25 Ovcon-35® norethindrone 0.40 Brevicon® norethindrone 0.50 Modicon® norethindrone 0.50 Necon® norethindrone 1.00 Norethin® norethindrone 1.00 Norinyl® 1/35 norethindrone 1.00 Ortho-Novum® 1/35 norethindrone 1.00 Demulen® 1/35 ethynodiol diacetate 1.00 Zovia® 1/35E ethynodiol diacetate 1.00 phasic Ortho-Novum® norethindrone 0.50/1.00 35/35 mcgm 10/11 Jenest® norethindrone 0.50/1.00 phasic Ortho-Tri-Cyclen® norgestimate 0.15/0.215/0.25 35/35/35 mcgm Ortho-Novum® norethindrone 0.50/0.75/1.00 7/7/7 Tri-Norinyl® norethindrone 0.50/1.00/0.50 50 mcgm Necon® 1/50 norethindrone 1.00 Norinyl® 1/50 norethindrone 1.00 Ortho-Novum® 1/50 norethindrone 1.00 Ovcon-50® norethindrone 1.00 Ovral® norgestrel 0.50 Demulen® 1/50 ethynodiol diacetate 1.00 Zovia® 1/50E ethynodiol diacetate 1.00 Which pills have higher progestin side efects or cause more acne and hair growth? Each progestin has a diferent potency, milligram per milligram, in terms of progesterone efect to stop menstrual bleeding or androgen efect to stimulate acne and hair growth. -

Etats Rapides
List of European Pharmacopoeia Reference Standards Effective from 2015/12/24 Order Reference Standard Batch n° Quantity Sale Information Monograph Leaflet Storage Price Code per vial Unit Y0001756 Exemestane for system suitability 1 10 mg 1 2766 Yes +5°C ± 3°C 79 ! Y0001561 Abacavir sulfate 1 20 mg 1 2589 Yes +5°C ± 3°C 79 ! Y0001552 Abacavir for peak identification 1 10 mg 1 2589 Yes +5°C ± 3°C 79 ! Y0001551 Abacavir for system suitability 1 10 mg 1 2589 Yes +5°C ± 3°C 79 ! Y0000055 Acamprosate calcium - reference spectrum 1 n/a 1 1585 79 ! Y0000116 Acamprosate impurity A 1 50 mg 1 3-aminopropane-1-sulphonic acid 1585 Yes +5°C ± 3°C 79 ! Y0000500 Acarbose 3 100 mg 1 See leaflet ; Batch 2 is valid until 31 August 2015 2089 Yes +5°C ± 3°C 79 ! Y0000354 Acarbose for identification 1 10 mg 1 2089 Yes +5°C ± 3°C 79 ! Y0000427 Acarbose for peak identification 3 20 mg 1 Batch 2 is valid until 31 January 2015 2089 Yes +5°C ± 3°C 79 ! A0040000 Acebutolol hydrochloride 1 50 mg 1 0871 Yes +5°C ± 3°C 79 ! Y0000359 Acebutolol impurity B 2 10 mg 1 -[3-acetyl-4-[(2RS)-2-hydroxy-3-[(1-methylethyl)amino] propoxy]phenyl] 0871 Yes +5°C ± 3°C 79 ! acetamide (diacetolol) Y0000127 Acebutolol impurity C 1 20 mg 1 N-(3-acetyl-4-hydroxyphenyl)butanamide 0871 Yes +5°C ± 3°C 79 ! Y0000128 Acebutolol impurity I 2 0.004 mg 1 N-[3-acetyl-4-[(2RS)-3-(ethylamino)-2-hydroxypropoxy]phenyl] 0871 Yes +5°C ± 3°C 79 ! butanamide Y0000056 Aceclofenac - reference spectrum 1 n/a 1 1281 79 ! Y0000085 Aceclofenac impurity F 2 15 mg 1 benzyl[[[2-[(2,6-dichlorophenyl)amino]phenyl]acetyl]oxy]acetate -

Effects of 2 Mg Chlormadinone Acetate/0.03 Mg Ethinylestradiol In
Journal für Reproduktionsmedizin und Endokrinologie – Journal of Reproductive Medicine and Endocrinology – Andrologie • Embryologie & Biologie • Endokrinologie • Ethik & Recht • Genetik Gynäkologie • Kontrazeption • Psychosomatik • Reproduktionsmedizin • Urologie Effects of 2 mg Chlormadinone Acetate/0.03 mg Ethinylestradiol in Primary Dysmenorrhoea: The BEDY (Belara(R) Evaluation on Dysmenorrhea) Study - an Open Non-Comparative, Non-Interventional Observational Study with 4,842 Women Schramm GAK, Waldmann-Rex S J. Reproduktionsmed. Endokrinol 2010; 7 (Sonderheft 1), 112-118 www.kup.at/repromedizin Online-Datenbank mit Autoren- und Stichwortsuche Offizielles Organ: AGRBM, BRZ, DVR, DGA, DGGEF, DGRM, D·I·R, EFA, OEGRM, SRBM/DGE Indexed in EMBASE/Excerpta Medica/Scopus Krause & Pachernegg GmbH, Verlag für Medizin und Wirtschaft, A-3003 Gablitz Effects of CMA/EE in Primary Dysmenorrhoea Effects of 2 mg Chlormadinone Acetate/ 0.03 mg Ethinylestradiol in Primary Dysmenorrhoea: The BEDY (Belara® Evaluation on Dysmenorrhea) Study – an Open, Non-Comparative, Non-Interventional Observational Study with 4,842 Women G. A. K. Schramm, S. Waldmann-Rex Background: This prospective, non-interventional, observational study designed to reflect the daily medical practice which is very important for the product observation liability investigated the effects of 2.0 mg chlormadinone acetate/0.03 mg ethinylestradiol (CMA/EE) on dysmenorrhoea and related problems. Study design: A total of 4,842 patients were observed during a six-cycle period (26,945.5 treatment cycles) in 608 office-based gynaecological centres throughout Germany. Results: The administration of CMA/EE significantly reduced the number of patients who suffered from menstrual pain (–50.4 %). Analgesic use and absenteeism from school or work due to dysmenorrhoea was reduced by 74.6 % in OC starters and 91.9 % in OC switchers. -

Minutes of PRAC Meeting on 09-12 July 2018
6 September 2018 EMA/PRAC/576790/2018 Inspections, Human Medicines Pharmacovigilance and Committees Division Pharmacovigilance Risk Assessment Committee (PRAC) Minutes of the meeting on 09-12 July 2018 Chair: June Raine – Vice-Chair: Almath Spooner Health and safety information In accordance with the Agency’s health and safety policy, delegates were briefed on health, safety and emergency information and procedures prior to the start of the meeting. Disclaimers Some of the information contained in the minutes is considered commercially confidential or sensitive and therefore not disclosed. With regard to intended therapeutic indications or procedure scope listed against products, it must be noted that these may not reflect the full wording proposed by applicants and may also change during the course of the review. Additional details on some of these procedures will be published in the PRAC meeting highlights once the procedures are finalised. Of note, the minutes are a working document primarily designed for PRAC members and the work the Committee undertakes. Note on access to documents Some documents mentioned in the minutes cannot be released at present following a request for access to documents within the framework of Regulation (EC) No 1049/2001 as they are subject to on- going procedures for which a final decision has not yet been adopted. They will become public when adopted or considered public according to the principles stated in the Agency policy on access to documents (EMA/127362/2006, Rev. 1). 30 Churchill Place ● Canary Wharf ● London E14 5EU ● United Kingdom Telephone +44 (0)20 3660 6000 Facsimile +44 (0)20 3660 5555 Send a question via our website www.ema.europa.eu/contact An agency of the European Union © European Medicines Agency, 2018. -
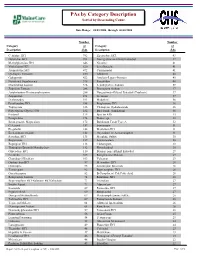
Report 752 by Category Description
PAs by Category Description Sorted by Descending Count Date Range: 04/01/2006 through 06/30/2006 Number Number Category of Category of Description PAs Description PAs Cetirizine HCl 792 Ziprasidone HCl 43 Duloxetine HCl 784 Norelgestromin-Ethinyl Estradiol 42 Methylphenidate HCl 646 Nicotine 41 Venlafaxine HCl 620 Levofloxacin 41 Atomoxetine HCl 472 Carisoprodol 41 Quetiapine Fumarate 430 Albuterol 40 Gabapentin 422 Amylase-Lipase-Protease 40 Nutritional Supplements 378 Famotidine 40 Montelukast Sodium 326 Levothyroxine Sodium 39 Zolpidem Tartrate 288 Enoxaparin Sodium 37 Amphetamine-Dextroamphetamine 286 Norgestimate-Ethinyl Estradiol (Triphasic) 37 Aripiprazole 271 Tretinoin 37 Desloratadine 191 Modafinil 36 Fexofenadine HCl 188 Pioglitazone HCl 36 Topiramate 186 Citalopram Hydrobromide 36 Polyethylene Glycol 3350 182 Budesonide (Inhalation) 36 Fentanyl 175 Epoetin Alfa 33 Eszopiclone 174 Etanercept 33 Esomeprazole Magnesium 172 Botulinum Toxin Type A 32 Celecoxib 157 Somatropin 31 Pregabalin 148 Metformin HCl 31 Escitalopram Oxalate 142 Oxycodone w/ Acetaminophen 31 Sertraline HCl 135 Morphine Sulfate 30 Risperidone 127 Levetiracetam 30 Bupropion HCl 118 Clonazepam 30 Tiotropium Bromide Monohydrate 112 Phenobarbital 30 Oxycodone HCl 110 Drospirenone-Ethinyl Estradiol 29 Ezetimibe 105 Rosiglitazone Maleate 29 Clopidogrel Bisulfate 103 Valsartan 29 Ondansetron HCl 97 Memantine HCl 28 Olanzapine 93 Sumatriptan Succinate 28 Temazepam 92 Buprenorphine HCl 28 Oxcarbazepine 82 B-Complex w/ C & Folic Acid 28 Rabeprazole Sodium 74 Ranitidine -

Oral Contraceptives
ORAL CONTRACEPTIVES STRENGTH DRUG ESTROGEN PROGESTIN (ESTROGEN/PROGESTIN) MONOPHASIC Aviane 28, Lessina, Lutera, Orsythia, Ethinyl estradiol Levonorgestrel 20mcg/0.1mg Sronyx† Beyaz*, YAZ Ethinyl estradiol Drospirenone 20mcg/3mg [Gianvi, Loryna, Vestura]† Brevicon, Modicon Ethinyl estradiol Norethindrone 35mcg/0.5mg [Necon 0.5/35, Nortrel 0.5/35]† Desogen Ethinyl estradiol Desogestrel 30mcg/0.15mg [Apri, Emoquette, Reclipsen, Solia]† Femcon Fe, Ovcon 35 Ethinyl estradiol Norethindrone 35mcg/0.4mg [Balziva, Briellyn, Philith, Vyfemla Zenchent, Zenchent Fe]† Loestrin 21 1/20, Loestrin Fe 1/20, Ethinyl estradiol Norethindrone acetate 20mcg/1mg Loestrin 24 Fe, Minastrin 24 Fe [Gildess Fe 1/20, Junel 1/20, Junel Fe 1/20, Larin 1/20, Larin Fe 1/20, Microgestin 1/20, Microgestin Fe 1/20]† Loestrin 21 1.5/30, Loestrin Fe 1.5/30 Ethinyl estradiol Norethindrone acetate 30mcg/1.5mg [Gildess Fe 1.5/30, Junel 1.5/30, Junel Fe 1.5/30, Microgestin 1.5/30, Microgestin Fe 1.5/30]† Lo/Ovral Ethinyl estradiol Norgestrel 30mcg/0.3mg [Cryselle, Low-Ogestrel]† Lybrel Ethinyl estradiol Levonorgestrel 20mcg/0.09mg [Amethyst]† [Altavera, Levora, Marlissa, Portia]† Ethinyl estradiol Levonorgestrel 30mcg/0.15mg Norinyl 1/35, Ortho-Novum 1/35 Ethinyl estradiol Norethindrone 35mcg/1mg [Alyacen 1/35, Cyclafem 1/35, Dasetta 1/35, Necon 1/35, Nortrel 1/35]† Necon 1/50, Norinyl 1/50 Mestranol Norethindrone 50mcg/1mg Ogestrel 0.5/50† Ethinyl estradiol Norgestrel 50mcg/0.5mg Ortho-Cyclen Ethinyl estradiol Norgestimate 35mcg/0.25mg [MonoNessa, Previfem, Sprintec]† Ovcon