Iso 50001:2018
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Ashtavinayaka Temples, the Yatra Vidhi and More
Newsletter Archives www.dollsofindia.com Ashtavinayaka - the Eight Holy Abodes of Ganesha Copyright © 2018, DollsofIndia Sri Ganesha, also known as Vinayaka, is one of the most popular deities of the Hindu pantheon. Highly revered as the Harbinger of Success and the Remover of Obstacles, this Elephant- Headed son of Shiva and Parvati is venerated not only by Hindus, but also by people from all religions and all walks of life; from all over the world. One can find innumerable Ganesha temples all over the globe. In fact, all Hindu temples; irrespective of who the main deity is; necessarily have at least one shrine dedicated to Vighnavinayaka. Devotees first visit this shrine, pray to Ganesha to absolve them of their sins and only then proceed to the main sanctum. So exalted is the position of this God in Hindu culture. Shola Pith Ganapati Sculpture There are eight forms of Vinayaka, collectively referred to as Ashtavinayaka ('Ashta' in Sanskrit means 'eight'). The Ashtavinayaka Yatra implies a pilgrimage to the eight Vinayaka temples, which can be found in the Indian State of Maharashtra, situated in and around the city of Pune. The Yatra follows a particular route, in a pre-ascertained sequence. Each of these ancient Ashtavinayaka temples features a distinct murti (idol) of Ganesha and has a different legend behind its existence. Not only that; the appearance of each murti; even the angle of his trunk; are all distinct from one another. In this post, we bring you all the details on the Ashtavinayaka temples, the Yatra vidhi and more. Resin Ashtavinayak with Shloka on Wood - Wall Hanging The Ashtavinayaka Temples The eight temples of Ashtavinayaka, in their order, are as follows: 1. -

[email protected]@Aartigroup.Com 2 20 MICRONS LTD 0888015356 20 MICRONS LTD
Sr. No. Importer's Name IEC ADDRESS E. Mail 1 AARTI INDUSTRIES LTD 0389029971 UDYOG KSHETRA, 2ND FLOOR, MULUND-GOREGAON LINK ROAD, MULUND, MUMBAI, MAHARASHTRA PIN-400080 [email protected]@aartigroup.com 2 20 MICRONS LTD 0888015356 20 MICRONS LTD. 307 - 308 ARUNDEEP COMPLEX, RACE COURSE SOUTH, B.A. ROAD BARODA ,GUJRAT PIN-390007 [email protected] 3 3M INDIA LIMITED 0793012112 48-51,ELECTRONICS CITY HOSUR ROAD BANGALORE/KARNATAKA PIN-562158 [email protected], [email protected] 4 A CUBE TECHNO INDUSTRIES 0516968491 PLOT NO 81, GALI NO 1,RAJIV COLONY GURGAON,HARYANA PIN-122001 [email protected] 5 A V H POLYCHEM PVT LTD 309065852 B-101, FIRST FLOOR, GIRIRAJ HEIGHTS, ABOVE AXIS BANK, VRAJBHUMI LINK ROAD, KANDIVAL (W), MUMBAI- 400067 [email protected] 6 A-1 FENCE PRODUCTS CO. PVT. LTD. 303080311 21, RAJU INDUSTRIAL ESTATE, PENKAR PADA ROAD, NEAR DAHISAR CHECK NAKA MIRA DIST THANE , MUMBAI MAHARASHTRA 401104 [email protected] 7 AADITYA AGRO IMPEX 816917612 201, M.V.HOUSE,OPP.HATHISINGS JAIN ,TEMPLE,OS.DELHI GATE,SHAHIBAUG, AHMEDABAD,GUJARAT PIN-380004 [email protected] 8 AAREL IMPORT EXPORT PVT. LTD. 312023227 A-2, UNIT NO.29, GRD.FLR, SHAH AND NAHAR INDL.ESTATE, DHANRAJ MILL COMPOUND,LOWER PAREL, MUMBAI PIN-400013 [email protected] 9 AARTI DRUGS LIMITED 0388189151 AARTI DRUGS LIMITED MAHENDRA INDL.ESTATE III FLR. PLOT ,NO.109 D ROAD NO.29 SION E MUMBAI,MAHARASHTRA PIN-400022 [email protected] 10 AASHRAY TRADING INDIA LLP 5016902020AASHRAY TRADING INDIA LLP NAKODA FOOD INDUST 239,SOUTH OLD B AGADGANJ SMALL FACTORY NAGPUR,MAHARASHTRA PIN-440008 [email protected] 11 AAYUSH IMPEX 0300068573 AAYUSH IMPEX SHOP NO.F-28/29, APMC FRUIT MARKET, SECTOR-19, TURBHE VASHI, NAVI MUMBAI, MAHARASHTRA PIN-400703 [email protected] 12 ABACUS PERIPHERALS PVT LTD 0396051731ABACUS PERIPHERALS PVT. -

Ranjangaon Ganpati Trust
F. Y. 2012-13 A.Y. 2013-14 SHREE KSHETRA RANJANGAON GANPATI DEVSTHAN TRUST SCHEDULE-"A" EXPENDITURE IN RESPECT OF PROPERTY PARTICULARS AMOUNT Repairs and maintanance 1,160,015.00 Insurance 429,208.00 Rent Taxes & Cess 9,894.00 TOTAL RS 1,599,117.00 F. Y. 2012-13 A.Y. 2013-14 SCHEDULE-"B" ESTABLISHMENT EXPENSES PARTICULAR AMOUNT Accounts Written Off 9,396.00 Advertisement 169,998.00 Bank charges 5,632.00 ISO Certification Charges 16,854.00 Legal Expenses 105,000.00 Meeting Allowance Expenses 52,500.00 Miscellaneous Expenses 27,365.00 News Papers and Periodicals 9,276.00 Postage and stamp expenses 3,060.00 Printing and stationary 264,518.00 Professional Fees 89,158.00 Security and Cleaning Service Expenses 4,444,975.00 Sweeping and Cleaning Expenses 161,974.00 Tea and Welfare Expenses 88,808.00 Telephone Charges 69,444.00 Travelling and Conveyance 111,963.00 TOTAL RS 5,629,921.00 F. Y. 2012-13 A.Y. 2013-14 SCHEDULE "C" EXPENDITURE ON OBJECT OF THE TRUST PARTICULAR AMOUNT Empolyees salary and allowances Ex-Gratia Expenses 198,250.00 Provident Fund Employer's Contribution 124,299.00 Salary and Wages Expenses 2,360,898.00 Trainning and Seminar Expenses 4,550.00 Worker Walefare Expenses 286,511.00 Sub Total (a) 2,974,508.00 Welfare Expenses Ranjangaon Grampanchayat Water Pipeline Contri 3,000,000.00 Welfare Expenses 29,926.00 Welfare for Schools Expenses 586,479.00 Welfare Free/Nominal Ambulance 114,463.00 Welfare- Medical Assistance for Poor People 399,139.00 Welfare - Public 271,800.00 Sub Total (b) 4,401,807.00 Yatra and Function Expenses Bhadrapad Yatra Expenses 1,461,759.00 Function and Festival Expenses 887,115.00 Ganesh Puran Utsav 552,592.00 Jeshthi Mangalmurti Yatra 80,038.00 Kirtan Expenses - Babamaharaj Satarkar 527,407.00 Magh Yatra Expenses 162,663.00 Mahaganpati Purashkar 1,146,319.00 Navratra Utsav Expenses 106,942.00 Ramayan Programme Expenses 1,209,574.00 Tripuri Pornima Expenses 60,170.00 Sub Total (c) 6,194,579.00 Abhishek Exps 98,867.00 Annachatra Expenses 2,234,333.00 Boiler Expenses 21,425.00 Diesel Expenses 48,708.00 Electricity Exp. -
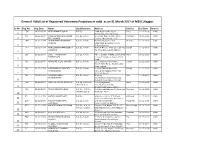
General Valid List of Registered Veterinary Practitioners Valid As on 31 March 2017 of MSVC,Nagpur
General Valid List of Registered Veterinary Practitioners valid as on 31 March 2017 of MSVC,Nagpur. Sr No Reg No Reg Date Name Qualifications Address District Due Date Remark 34 12-12-1974 APTE HEMANT GOPAL B.V.Sc. 1244,Apte Road,Deccan Pune 11-12-2019 Valid 1 Gymkhana, Pune-411004. 102 12-12-1974 MISS BHAGWAGAR SILOO B.V.Sc. & A.H. 2,Ferena, Opp.Fariyas Hotel, Mumbai 09-12-2019 Valid 2 ERUCHSHAW Colaba , Mumbai-400005. 144 12-12-1974 CHANDEKAR YESHWANT B.V.Sc. & A.H. Plot No.89,Kannamwar Wardha 11-12-2019 Valid MADHAV Nagar,Opp.Sonegaon Police 3 Station,Wardha 157 12-12-1974 CHOUDHARY RAMATEERTH B.V.Sc. & A.H. At/Post-Nandre Wale Lane,267-B, Sangli 11-12-2019 Valid BABURAO. Tal.Miraj,Dist.Sangli-416423 4 286 12-12-1974 DIXIT PRABHAKAR B.V.Sc. & A.H. Flat 4, Suman Prabha, 21 Krishna Pune 09-12-2019 Valid DATTATRAYA Colony Paramhans Nagar, Poud 5 Road, 332 12-12-1974 GHAGARE VILAS GOVIND B.V.Sc. & A.H. 12,Chandarani Apartment, Sangli 11-12-2019 Valid Dr.P.R.Patil Marg , Dr.Ambedkar 6 Road, Sangli. 369 12-12-1974 HARPANHALLI GANPATI B.V.Sc. & A.H. Keshav Smruti',Opp.Vita Sangli 11-12-2019 Valid NARSINGRAO Dairy,Brahmanpuri,Miraj-416 7 410,Dist.Sangli. 380 12-12-1974 INAMDAR ANANT B.V.Sc. & A.H. Bhartiya Pune 11-12-2019 Valid JAYARAMPANT Agro-Industries,Development 8 Research Foundation,Central 429 12-12-1974 JOSHI BHASKAR SHIVRAM B.V.Sc. -

BK Centers in Maharashtra, India
BK Centers in Maharashtra, India Source: “Centers Finder” service by www.bkgsu.com/centres Centre Area Centre Address Contact Info. Gadhinglaj H No: 1379/2, Rajyog Bhawan 02327- 223374 Azad Road 9423801228 Gadhinglaj [email protected] Kolhapur-416502 Maharashtra Halkarni Sadbhawna Bhawan, H No: 772 02327- 263967 Teli Galli 9503328299, Gadhinglaj 7776995765 Halkarni Kolhapur-416506 Maharashtra Latur Old Ausa Rajyog Bhavan 2382-200097, 200813 Road Old Ausa Road 9423076927 Tal-latur [email protected] Latur Latur-413531 Maharashtra Achalpur Camp Shiv Darshan Bhawan 07223- 221353 Brijdham Mandir Road 9422603310, Paratwada, Mohan Nagar 9405974303 Achalpur Camp [email protected] Amravati-444805 Maharashtra Ahmednagar Shiv Darshan, H.no: 63/40 0241- 2324058 Mahavir Nagar 7276430213 Savedi Road [email protected] Ahmednagar Ahmednagar-414001 Maharashtra Akola Kirti Tapasya Bhavan, H.no: I/3 0724- 2458547 Nagar Near R.t.o, Gorakshan Road 9922511718, Kirti Nagar 8275394025 Akola [email protected] Akola-444001 g Maharashtra Amalner Sadbhavana Bhawan, Brahma Kumaris Marg 02587-224159 76, Near Maratha Mangal Karyalaya 9860371118 Dadasaheb Deshmukh Nagar [email protected] Amalner Jalgaon-425401 Maharashtra Ambajogai Plot No: 5, Sadhana Dham 02446-247513 Om Shanti Colony 9421336765 Brahmakumaris Road [email protected] Ambajogai Beed-431517 Maharashtra Ambernath C-3 & 6, Ist Floor 0251-2604601 Shiv Basav Housing Society 9503274842 Shiv Mandir Road [email protected] Ambernath Thane-421501 Maharashtra Amravati Sukh Shanti Bhawan 0721-2574397 -

Student Allotment As Per Police Station for the Bandobast of Ganesh Festival-2012 (Training Not Attended) FEMALE VOLUNTEERS FARASKHANA POLICE STATION Sr.No
Student Allotment As per Police Station for the Bandobast of Ganesh Festival-2012 (Training Not Attended) FEMALE VOLUNTEERS FARASKHANA POLICE STATION Sr.No. Name of Student \ College \ Mobile No Address Work Place / Police Station 1 DHANASHREE RAJENDRA BADAMBE 2, SHIVRATNA SOCIETY, BIBVEWADI, BIBAWEWADI ROAD, PUNE CITY H. M. V. M 8237774874 HAVELI, PUNE, MAHARASHTRA, PIN- 411037 FARASKHANA 2 SOALI DNYANESHVAR SHELAR 970, VIGNAHAR APP, SADASHIV PETH, , HAVELI, PUNE, MANDAI HUZURPAGA MAHAVIDYALA (HMVM) 9960471330 MAHARASHTRA, PIN- 411003 FARASKHANA 3 VAISHALI SHANKAR SHAJWAL , NANDED PHATA, , SINHGAD ROAD, HAVELI, PUNE, FARASKHANA SIDDHIVINAYAK COLLEGE 9850987981 MAHARASHTRA, PIN- 411023 FARASKHANA 4 MADHURI BABURAO BHOSLE , SONAI BUNGLOW, SUKHSAGAR NAGAR, IN FRONT OF LAXMI ROAD VIT COLLEGE 7385499337 BSNL OFFICE, , PUNE, MAHARASHTRA, PIN- 411046 FARASKHANA TOTAL FEMALE VOLUNTEERS = (4 Volunteers) Tuesday, September 18, 2012 Developed by HC/WO Ingavale R N Page 1 of 28 Student Allotment As per Police Station for the Bandobast of Ganesh Festival-2012 (Training Not Attended) FEMALE VOLUNTEERS KHADAK POLICE STATION Sr.No. Name of Student \ College \ Mobile No Address Work Place / Police Station 1 AKSHTA JITENDRA BHOSALE 929, KIRAD HOSPITAL, NANA PETH, , HAVELI, PUNE, PUNE CITY APPASAHEB JEDHE ART. COM. SCI. COLLEGE MAHARASHTRA, PIN- 411002 KHADAK 2 ASHWINI ARJUN GOTE 739, A D CAMP CHOWK, NANA PETH, NANA PETH, HAVELI, PUNE CITY APPASAHEB JEDHE ART. COM. SCI. COLLEGE 8805523445 PUNE, MAHARASHTRA, PIN- 411002 KHADAK TOTAL FEMALE VOLUNTEERS = (2 Volunteers) Tuesday, September 18, 2012 Developed by HC/WO Ingavale R N Page 2 of 28 Student Allotment As per Police Station for the Bandobast of Ganesh Festival-2012 (Training Not Attended) FEMALE VOLUNTEERS DECCAN POLICE STATION Sr.No. -
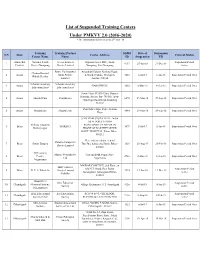
List of Suspended Training Centers Under PMKVY 2.0 (2016-2020) *The Information Below Is As on 29Th Oct ‘18
List of Suspended Training Centers Under PMKVY 2.0 (2016-2020) *The information below is as on 29th Oct ‘18 Training Training Partner SDMS Date of Suspension S.N State Center Address Current Status Center Name Name ID Suspension Till Arunachal National Youth Access Edutech Opposite Govt. PHC, Town Suspension Period 1 9137 27-Jun-18 27-Dec-18 Pradesh Project Nampong Private Limited Nampong, Post Nampong Active Power To Empower Amarabati Colony, Krishna Nagar, Chabua Kaushal 2 Assam Skills Private A-Ward, Chabua, Dibrugarh, 1210 6-Jul-17 6-Jan-18 Suspension Period Over Bikash Kendra Limited Assam- 786184 Scholars Academy Scholars Academy 3 Assam GAROGHULI, 3652 6-Mar-18 6-Sep-18 Suspension Period Over Education Trust Education Trust Town- Hajo, PO.PS- Hajo, District- Kamrup, Assam, Pin- 781102 Town- 4 Assam Shoptul.Com Shoptulcom 6095 29-Mar-18 29-Sep-18 Suspension Period Over Hajo Hajo Road Kiran Shopping 781102 Post Office Hajo, Police Station 5 Assam Shoptulcom Shoptul.com 4544 29-Mar-18 29-Sep-18 Suspension Period Over Hajo SHIV RAM UTSAV HALL 2nd & 3rd FLOOR,STATION Shiksha Eduskills ROAD,BAKHTIYARPUR IN 6 Bihar SHIKSHA 1099 5-Jul-17 5-Jan-18 Suspension Period Over Bakhtiyarpur FRONT OF BAKHTIYARPUR GOVT. HOSPITAL, Patna, Bihar - 803212 Near Adarsh colony, behind Datapro Computers 7 Bihar Siwan Datapro TumTun, bahu petrol bunk, Bihar - 1329 25-Aug-17 25-Feb-18 Suspension Period Over Private Limited 841226 4Q Learning Mosaic Network Pvt Gautam Budh Nagar, Post- 8 Bihar Centre, 2940 8-Mar-18 8-Sep-18 Suspension Period Over Ltd Nagarnausa Nagarnausa MAWAR COMPLEX, 2nd Floor,, at Skill Ventures Old G.T. -

WHO-GMP Certified Manufacturing Units for Certificate of Pharmaceutical Products (Copps) in Various States of India
WHO-GMP Certified Manufacturing Units for Certificate of Pharmaceutical Products (COPPs) in various states of India# S. No. Name of State Total no. of WHO GMP Certified Manufacturers 1. Andaman & Nicobar Island 0 2. Andhra Pradesh 73 3. Arunachal Pradesh 0 4. Assam 7 5. Bihar 0 6. Chandigarh 0 7. Chhattisgarh 0 8. Dadra & Nagar Haveli 3 9. Daman & Diu 31 10. Delhi 7 11. Goa 54* 12. Gujarat 684* 13. Haryana 35 14. Himachal Pradesh 202 15. Jammu & Kashmir 14 16. Jharkhand 0 17. Karnataka 73 18. Kerala 8 19. Lakshadweep 0 20. Madhya Pradesh 69* 21. Maharashtra 229 22. Manipur 0 23. Meghalaya 0 24. Mizoram 0 25. Nagaland 0 26. Orissa 0 27. Puducherry 29 28. Punjab 22 29. Rajasthan 27 30. Sikkim 32 31. Tamil Nadu 79 32. Telangana 172 33. Tripura 0 34. Uttar Pradesh 23 35. Uttrakhand 128 36. West Bengal 5 Total 2006 #data as received from States/UTs as on May 2019. *data including loan license. S. Sub- Name of the firm Address of the WHO-GMP certified manufacturers No. Sr.no. State- wise Andaman & Nicobar Island Nil Andhra Pradesh 1 1 M/s Laurus Labs Ltd, Plot No.21, JNPC, Parawada(M), Visakhapatnam 2 2 M/s Mylan Laboratories Unit-10, SEZ, JNPC , Parawada, Visakhapatnam Ltd, 3 3 M/s Gland Pharma Ltd, Plot No.49& 50, JNPC, Parawada(M), Visakhapatnam 4 4 M/s Biocon Ltd, Plot No. 02, JNPC, Parawada(M), Visakhapatnam 5 5 M/s Dr.Reddy’s FTO-VII, Phase-III, VSEZ, Duvvada, Visakhapatnam Laboratories, 6 6 M/s Vegesna Plot No.7, JNPC, Parawada(M), Visakhapatnam Laboratories Ltd, 7 7 M/s Azico Biophore India Plot No. -

District Census Handbook, Pune
CENSUS OF INDIA 1981 DISTRICT CENSUS HANDBOOK PUNE Compiled by THE MAHARASHTRA CENSUS DIRECTORATE BOMBAY PRINTED IN INDIA BY THE MANAGER, GOVERNMENT CENTRAL PRESS, BOMBAY AND PUBLISHED BY THE DIRECTOR, GOVERNMENT PRINTING, STATIONERY AND PUBLICATIONS, MAHARASHTRA STATE, BOMBAy-400 004. 1986 [Price Rs. 30.00] lLJ S c o "" « Z ;! ~a,'~,,_ ~ 0:: :::> ~ g ~ f -~, ~ Q. ~ 0 ~ ~ g :::r: ~ :z: ~ J- ~ § .! 0:: U <S ~ « ~ ::r: 0::: ~ ~ ~ « J- .j ~ 0 (J) ~ ~ ~ LO '5, ~ ~ :'! 'j' ~ 0 c- i '0 .g 02 ~ f:z: li ~~~ti<!::':ZI- It p. (', P. I'- \) <t po. a:: ~ ..(. <t I>- ,. .-~ .;>~<:> <t '- /'\ i ' " U'l C,; \. \ "- i I,~ lC ~1({:;OJ.j , ~. ' .~ ..... .'. a.~ u I/) a:: i' 0 " Ul (' <,' 0 ~/1·...... ·"r·j ,,r-./ C> .tV /& 1'-" , .IS 10 c, It 0- '\. ". ""• ~ :; ,F \. ') V ij c 0 ~ <Il~ MOTIF Shaniwar Wada, once upon a time was the palace of Peshwas (chief executives of the Maratha Kingdom founded by Chhatrapati Shivaji). Peshwas went on conquering places as far as Panipat and Atak and extending the boundaries of the kingdom beyond Chambal river in North India. This finest palace of the time (1730-1818) but now in dilapidated condition is in the heart of the Pune city. This palace as it finally stood was a seven-storeyed building with four large and several small courts some of which were named as Ganapati Rang Mahal, Nach Diwankhana, Arse Maltal, Hastidanti Mahal. Today, the remains (enclosure, plinths and the surrounding wall) are mute witnesses of the past Maratha glory. CENSUS OF INDIA 1981 SERIES 12-MAHARASHTRA DISTRICT PUNE ERRATA SLIP Page Column No Item No. -

HIGH COURT of BOMBAY WEBSITE and E-MAIL ADDRESSES ______Website Address : E-MAIL ADDRESS : [email protected]
HIGH COURT OF BOMBAY WEBSITE AND E-MAIL ADDRESSES ________________________________________________________________ Website Address : www.bombayhighcourt.nic.in E-MAIL ADDRESS : [email protected] Registrar General & Registrars E-mail Addresses Registrar General : [email protected] Registrar/Principal Secretary : [email protected] to the Hon'ble the Chief Justice Registrar (Vigilance-I) : [email protected] Registrar (Vigilance-II) : [email protected] Registrar (Inspection-I) : [email protected] Registrar (Inspection-II) : [email protected] Registrar (Judicial-I) : [email protected] Registrar (Judicial-II) : [email protected] Registrar (Legal & Research) : [email protected] Central Project Co-ordinator : [email protected] Registrar (Finance & Budget) : [email protected] [email protected] Registrar (Personnel) : [email protected] Registrar/Prothonotary & : [email protected] Senior Master Registrar Original Side : [email protected] (i) BOMBAY HIGH COURT TELEPHONE DIRECTORY - 2021 HIGH COURT OF JUDICATURE AT BOMBAY, MUMBAI - 400 032 INDIA I.S.D. CODE : 091 MUMBAI S.T.D. CODE NO. : 022 EPABX NOS. : 22673568, 22677066, 22682089, 22672001, 22673468, 22673569, 22673618, 22673235, 22670869, 22642268, 22642269 C.T.O. : 22682138, 22682137 I.V.R.S. NO. : 22673090 CONFERENCE HALL : 22648910, 22648810, 22648340 & ISDN LINE NOS. 22648890 FAX NOS. : 22624358, 22692439, 22673619, 22682071 (Inspection) NIC Computer Cell : 22676751 G.T. Hospital High Court No. : 22642267 Record Section, High Court, Small Causes Court Building : 22019425 C.T.O. Building : 22682137, 22682138, 1701 (EPABX) NAGPUR BENCH, NAGPUR-440 001 S.T.D. : 0712 FAX NO. : 2560280 EPABX NOS.: : 2562279, 2561102 I.S.D.N. LINE NOS. : 2564689, 2562083, 2562084 & 2562461 NIC COMPUTER CELL : 2544366 & 2540695 E-MAIL ADDRESS : [email protected] I.V.R.S. -

2015-16 As on 15.01.2017 Pursuant to Section 124(2) of the Companies Act, 2013 Due for Sl
List of shareholders - Unclaimed / Unpaid Dividend amount for the year 2015-16 as on 15.01.2017 pursuant to Section 124(2) of the Companies Act, 2013 Due for Sl. No. First Name Middle Name Last Name Address State District Pin Code Folio No. DP ID / Client ID Amount trasnfer to IEPF 1 ASHA PRASAD VI/I INCOME TAX & CENTRAL EXCUSE COLONY SHAPURA BHOPAL CALCUTTA WEST BENGAL KOLKATA PBES027644 3200.00 21-OCT-2023 2 SANDEEP AGGARWAL H NO 104 SECTOR 17 TEH GURGAON GURGAON HARYANA HARYANA GURGAON 122001 IN300214-IN300214-11855941 4000.00 21-OCT-2023 3 ABBAS ALI 37 BADA BHOIWADA UDAIPUR RAJASTHAN RAJASTHAN UDAIPUR 313001 PBES800114 2000.00 21-OCT-2023 4 JAYENDRA KUMAR RAMMOHAN SARDAR NAGAR MAIN ROAD RAJKOT GUJARAT RAJKOT 360001 PBES800245 4000.00 21-OCT-2023 5 ROHIT SHARMA 22 CARMEL APARTMENTS 30 NAPEAN SEA ROAD MUMBAI MAHARASHTRA MUMBAI 400006 PBES800117 2000.00 21-OCT-2023 6 UNIT TRUST OFINDIA NO 17 MERCHANTS CHAMBERS SIR VITHALDAS THACKERSEY MARG POST BAG NO 11410 BOMBAY MAHARASHTRA MUMBAI 400020 PBES019603 2000.00 21-OCT-2023 7 DSP MERRILL LYNCHLIMITED TULASIANI CHAMBERS 11TH FLOOR WEST WING 212 BACK BAY RECLAMATION MUMBAI MAHARASHTRA MUMBAI 400021 PBES019593 2000.00 21-OCT-2023 8 SBI CAP MKTLTDTHE STOCK HOLDING CORPORATION OF INDIA LTD 224 MITTAL COURT B WING 2ND FLOOR NARIMAN POINT BOMBAY MAHARASHTRA MUMBAI 400021 PBES021736 2800.00 21-OCT-2023 9 FLEMING FUND MANAGEMENTLUXEMBOURGSADEUTSCHE BANK AG GROUND FLOOR MHATRE PEN BLDG TULSI PIPE ROAD DADAR (W) MUMBAI MAHARASHTRA MUMBAI 400028 PBES022092 2800.00 21-OCT-2023 10 NATIONAL SECURITIES -
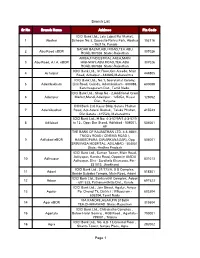
Branch List Page 1
Branch List Sr No Branch Name Address Pin Code ICICI Bank Ltd., Lala Lajpat Rai Market, 1 Abohar Scheem No.3, Opposite Nehru Park, Abohar 152116 - 152116, Punjab SADAR BAZAR,ABU ROAD,TEH.ABU 2 Abu Road eBOR 307026 ROAD,307026 State:-Rajasthan AMBAJI INDUSTRIAL AREA,MAIN 3 Abu Road, A.I.A. eBOR HIGHWAY,ABU ROAD,TEH.ABU 307026 ROAD,307026 State:-Rajasthan ICICI Bank Ltd., Ist Floor,Gm Arcade, Main 4 Achalpur 444805 Road, Achalpur - 444805,Maharashtra ICICI Bank Ltd., No.1, Secretariat Colony, 5 Adambakkam Link Road, Guindy, Adambakkam - 600088, 600088 Kancheepuram Dist., Tamil Nadu. ICICI Bank Ltd., Shop No - 2,Additional Grain 6 Adampur Market,Mandi,Adampur - 125052, Hissar 125052 Dist., Haryana ICICI Bank Ltd, Kasar Bldg, Satara Phaltan 7 Adarkibudruk Road, A/p Adarki Budruk, Taluka Phaltan, 415523 Dist Satara - 415523, Maharashtra ICICI Bank Ltd., H No: 4-3-57/9A/1,4-3-57/9 8 Adilabad to 12 , Opp: Bus Stand, Adilabad - 504001, 504001 AP THE BANK OF RAJASTHAN LTD. 4-3-168/1, TNGO's ROAD ( CINEMA ROAD ), 9 Adilabad eBOR HAMEEDPURA (DWARKANAGAR), Opp 504001 SRINIVASA HOSPITAL, ADILABAD - 504001 State:-Andhra Pradesh ICICI Bank Ltd., Suman Tower, Main Road, Adityapur, Kandra Road, Opposite AIADA 10 Adityapur 831013 Adityapur, Dist - Saraikela Kharsawa. Pin - 831013. Jharkhand ICICI Bank Ltd , 21/173/A, S S Complex, 11 Adoni 518301 Beside Saibaba Temple, Main Road, Adoni ICICI Bank Ltd., Sankarathil Complex, Adoor 12 Adoor 691523 - 691 523, Pathanamthitta Dist., Kerala ICICI Bank Ltd., Jain Street, Agalur, Aviyur 13 Agalur Po, Chengi Tk, District : Villupuram - 603204 603204, Tamil Nadu VIA KANORE,AGAR,PIN 313604 14 Agar eBOR 313604 TEH.DHARIAWAD State:-Rajasthan ICICI Bank Ltd., Chitrakatha Complex , 15 Agartala Below Hotel Somraj , HGB Road , Agartala - 799001 799001 , Tripura ICICI Bank Ltd., No.