Geological and Pleistocene Glaciations Explain the Demography and Disjunct Distribution of Red Panda (A
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Rapid Climate Vulnerability Assessment of Gangtok, Sikkim
February, 2018 RAPID CLIMATE VULNERABILITY ASSESSMENT OF GANGTOK, SIKKIM Developing Disaster Resilience Action Plan Through GIS & Prioritising Actions for Natural Disaster Risk Reduction in Urban Agglomerations of Shillong & Gangtok Gangtok City, Sikkim Gangtok, the capital city of Sikkim, is located in the eastern Himalayan range. The city is flanked on east and west by two streams, namely Roro Chu and Ranikhola, respectively, comprising 17 municipal wards. These two rivers divide the natural drainage into two parts, the eastern and western parts. Fig 1: Gangtok City map Gangtok City Characteristics Indicators Characteristics Classification of the city Hill Location 27°20’N 88°37’E Area 19.016 sq.km Climate Type Subtropical highland climate Temperature Average Annual Maximum Temperature - 27°C Average Annual Minimum Temperature - 19°C Rainfall Average annual : 3494 mm Height above Mean Sea Level 1,676 m above MSL Fig2: The main road connecting Gangtok to other cities Fig3: Gangtok M G Marg and towns Steep slopes, vulnerability to landslides, large forest cover and inadequate access to most areas characterize Gangtok. Unplanned urbanization and rapid construction on the hill slopes has increased the risk of environmental degradation in Gangtok. Hazard Exposure Sl. No Hazard Type Exposure 1 Flash Flood Y 2 Drought/ Heat Wave N 3 Earthquakes Y 4 Landslides Y 5 Forest Fires Y 6 Heavy Rainfall Y 7 Hailstorms/thundering Y Hazard Timeline Index Jan Feb Mar Apr May Jun Jul Aug Sept Oct Nov Dec Landslides Flash Flood Hailstorm/thundering Forest -

Probabilistic Travel Model of Gangtok City, Sikkim, India FINAL.Pdf
European Journal of Geography Volume 4, Issue2: 46-54, 2013 © Association of European Geographers ANALYSIS OF TOURISM ATTRACTIVENESS USING PROBABILISTIC TRAVEL MODEL: A STUDY ON GANGTOK AND ITS SURROUNDINGS Suman PAUL Krishnagar Govt. College, Department of Geography Nadia, West Bengal, India. Pin-741101 http://www.krishnagargovtcollege.org/ [email protected] Abstract: Tourism is now one of the largest industries in the world that has developed alongside the fascinating concept of eco-tourism. The concept of tourism could be traced back to ancient times when people travelled with a view to acquiring knowledge of unknown lands and people, for the development of trade and commerce, for religious preaching and also for the sheer adventure of discovery. In fact the system of tourism involves a combination of travel, destination and marketing, which lead to a process of its cultural dimension. Gangtok as a core centre of Sikkim has potential command area over different tourist spots in East Sikkim, which are directly linked by a network of roads centering Gangtok and are perfectly accessible for one-day trips. The tourist attractions of East Sikkim are clustered mostly in and around Gangtok, the state capital. This study shows the tourism infrastructure as well as seasonal arrival of tourists in the Gangtok city and to develop the probabilistic travel model on the basis of tourist perception which will help the tourism department for the further economic development of the area. KeyWords: Eco-tourism, command area, tourist attractions, probabilistic travel model 1. INTRODUCTION Tourism is now one of the largest industries in the world that has developed alongside the fascinating concept of eco-tourism. -

Status of Insectivorous Plants in Northeast India
Technical Refereed Contribution Status of insectivorous plants in northeast India Praveen Kumar Verma • Shifting Cultivation Division • Rain Forest Research Institute • Sotai Ali • Deovan • Post Box # 136 • Jorhat 785 001 (Assam) • India • [email protected] Jan Schlauer • Zwischenstr. 11 • 60594 Frankfurt/Main • Germany • [email protected] Krishna Kumar Rawat • CSIR-National Botanical Research Institute • Rana Pratap Marg • Lucknow -226 001 (U.P) • India Krishna Giri • Shifting Cultivation Division • Rain Forest Research Institute • Sotai Ali • Deovan • Post Box #136 • Jorhat 785 001 (Assam) • India Keywords: Biogeography, India, diversity, Red List data. Introduction There are approximately 700 identified species of carnivorous plants placed in 15 genera of nine families of dicotyledonous plants (Albert et al. 1992; Ellison & Gotellli 2001; Fleischmann 2012; Rice 2006) (Table 1). In India, a total of five genera of carnivorous plants are reported with 44 species; viz. Utricularia (38 species), Drosera (3), Nepenthes (1), Pinguicula (1), and Aldrovanda (1) (Santapau & Henry 1976; Anonymous 1988; Singh & Sanjappa 2011; Zaman et al. 2011; Kamble et al. 2012). Inter- estingly, northeastern India is the home of all five insectivorous genera, namely Nepenthes (com- monly known as tropical pitcher plant), Drosera (sundew), Utricularia (bladderwort), Aldrovanda (waterwheel plant), and Pinguicula (butterwort) with a total of 21 species. The area also hosts the “ancestral false carnivorous” plant Plumbago zelayanica, often known as murderous plant. Climate Lowland to mid-altitude areas are characterized by subtropical climate (Table 2) with maximum temperatures and maximum precipitation (monsoon) in summer, i.e., May to September (in some places the highest temperatures are reached already in April), and average temperatures usually not dropping below 0°C in winter. -

Khangchendzonga National Park
ASIA / PACIFIC KHANGCHENDZONGA NATIONAL PARK INDIA Sacred site in the Khangchendzonga National Park - © IUCN Tilman Jaeger India - Khangchendzonga National Park WORLD HERITAGE NOMINATION – IUCN TECHNICAL EVALUATION KHANGCHENDZONGA NATIONAL PARK (INDIA) – ID 1513 IUCN RECOMMENDATION TO WORLD HERITAGE COMMITTEE: To inscribe the property under natural criteria. Key paragraphs of Operational Guidelines: Paragraph 77: Nominated property meets World Heritage criteria. Paragraph 78: Nominated property meets integrity and protection and management requirements. 1. DOCUMENTATION Kangchenjunga Transboundary Conservation and Development Initiative in the Hindu Kush Himalayas. a) Date nomination received by IUCN: 16 March Prepared for TBPA. Krishna AP, Chhetri S, Singh KK 2015 (2002) Human Dimensions of Conservation in the Khangchendzonga Biosphere Reserve: The Need for b) Additional information officially requested from Conflict Prevention. Mountain Research and and provided by the State Party: Khangchendzonga Development 22(4):328-331. Lachungpa U (2009) National Park is nominated as a mixed site. ICOMOS Indigenous Lifstyles and Biodiversity Conservation wrote to the State Party in September, 2015 Issues in North Sikkim. Indian Journal of Traditional requesting supplementary information on a range of Knowledge 8(1): 51-55. Oli KP, Chaudhary S, Sharma issues related to the evaluation of cultural values. A UR (2013) Are Governance and Management Effective joint IUCN / ICOMOS progress report was then sent on within Protected Areas of the Kanchenjunga 17 December 2015 following the respective ICOMOS Landscape (Bhutan, India And Nepal)? PARKS 19(1): and IUCN Panel meetings. Requests were made of the 25-36. Sathyakumar S, Bashir T, Bhattacharya T, State Party to update the biodiversity inventory for Poudyal K (2011b) Mammals of the Khangchendzonga species within the property; consider changes to the Biosphere Reserve, Sikkim, India. -

INTRODUCTION 1 1 Lepcha Is a Tibeto-Burman Language Spoken In
CHAPTER ONE INTRODUCTION 11 Lepcha is a Tibeto-Burman language spoken in Sikkim, Darjeeling district in West Bengal in India, in Ilm district in Nepal, and in a few villages of Samtsi district in south-western Bhutan. The tribal home- land of the Lepcha people is referred to as ne mayLe VÎa ne máyel lyáng ‘hidden paradise’ or ne mayLe malUX VÎa ne máyel málúk lyáng ‘land of eternal purity’. Most of the areas in which Lepcha is spoken today were once Sikkimese territory. The kingdom of Sikkim used to com- prise all of present-day Sikkim and most of Darjeeling district. Kalim- pong, now in Darjeeling district, used to be part of Bhutan, but was lost to the British and became ‘British Bhutan’ before being incorpo- rated into Darjeeling district. The Lepcha are believed to be the abo- riginal inhabitants of Sikkim. Today the Lepcha people constitute a minority of the population of modern Sikkim, which has been flooded by immigrants from Nepal. Although the Lepcha themselves estimate their number of speakers to be over 50,000, the total number is likely to be much smaller. Accord- ing to the 1991 Census of India, the most recent statistical profile for which the data have been disaggregated, the total number of mother tongue Lepcha speakers across the nation is 29,854. While their dis- tribution is largely in Sikkim and the northern districts of West Ben- gal, there are no reliable speaker numbers for these areas. In the Dar- jeeling district there are many Lepcha villages particularly in the area surrounding the small town of Kalimpong. -

Over View of Package Highlights Tour Itinerary Sikkim Darjeeling 4N/5D: Gangtok (2N), Darjeeling(2N) Package Costing &
Over View of Package • Package Name: Sikkim Darjeeling 4N/5D • Tour Route Gangtok(2N), Darjeeling(2N) • Duration: 4N /5D • Number of Pax : 2 Adults • Travel Dates : 1st April – 31st June 2021 Highlights • Gangtok Local City Tour • Tsomgo Lake & Baba Mandir • Darjeeling Local City Tour Tour Itinerary Sikkim Darjeeling 4N/5D: Gangtok (2N), Darjeeling(2N) Day1:NJP / IXB/Siliguri to Gangtok After Pick Up from IXB/NJP/Siliguri, start your wonderful vacation & proceed to Gangtok (5,410 ft) which is 120 km away and takes 4.5 hours approximately.After reaching Gangtok, check-in at the hotel. Overnight stay. Day2:Tsomgo Lake and New Baba Mandir After early breakfast, start for a full day excursion to Tsomgo lake (12,400ft) and New Baba Mandir (13,200ft). Please carry 3 passport size photos and photocopies of any valid Govt Photo ID proof of all travellers. School IDs/Birth certificate required for children. Please note Nathula pass (Indo Chinese border) situated at 14,500 ft. is an optional sightseeing point, which is not a part of this package. Special Permit is required for Nathula, is done on extra cost paid directly on the spot and is totally dependent on the issuance of permit by the Sikkim tourism Dept. It is closed for visitors on Mondays and Tuesdays There will be extra charges too. Permits may be granted on a clubbing basis. (Nathula Extra Rs 5,000/- per Cab if Permitted) Day3:Gangtok Half-Day City Tour & Transfer to Darjeeling Go for Gangtok half day sightseeing covering Bakthang Waterfalls, Institute of Handicraft, Flower Show, Chorten, Tibetology and Ropeway.& Transfer to Darjeeling Day4:Darjeeling city tour Wake up early in the morning (3:30am) to witness the sunrise from Tiger Hill (2,590 meters/13 KM/45 min). -

Food Security in North-East Region of India — a State-Wise Analysis
Agricultural Economics Research Review Vol. 28 (Conference Number) 2015 pp 259-266 DOI: 10.5958/0974-0279.2015.00041.5 Food Security in North-East Region of India — A State-wise Analysis A. Roy*, N.U. Singh, D.S. Dkhar, A.K. Mohanty, S.B. Singh and A.K. Tripathi ICAR Research Complex for North-Eastern Hill Region, Umiam - 793 103, Meghalaya Abstract With the adoption of high-yielding varieties of paddy, the foodgrains production has increased in North- East region of India. To estimate the growth performance of agriculture, time series data on area, production and productivity of foodgrains have been analysed for the period 1972-73 to 2011-12, which was divided into three decades, viz. 1982-83 to 1991-92 (I decade), 1992-93 to 2001-02 (II decade), 2002-03 to 2011- 12 (III decade) and overall period 1972-73 to 2011-12. During the overall period, among the states, Nagaland registered the highest significant growth in area, production and yield, followed by Arunachal Pradesh and Mizoram. All the NE states have shown positive growth rates in area, production and yield increase. The decomposition analysis of growth has suggested that sources of output growth were almost same in all the periods. During the first decade, the major contribution in the change of foodgrain production in the region was of area effect (74.8%), followed by yield effect (22.8%), whereas in all-India, the yield effect was 71 per cent. During the second period, the region had almost half sharer (50.3%) of area effect in food security, followed by yield effect (42.7%). -

Detailed Project Report National Adaptation Fund
DETAILED PROJECT REPORT ON MANAGEMENT OF ECOSYSTEM OF KAZIRANGA NATIONAL PARK BY CREATING CLIMATE RESILIENT LIVELIHOOD FOR VULNERABLE COMMUNITIES THROUGH ORGANIC FARMING AND POND BASED PISCICULTURE for NATIONAL ADAPTATION FUND ON CLIMATE CHANGE SUBMITTED TO MINISTRY OF ENVIRONMENT, FOREST & CLIMATE CHANGE, GOVERNMENT OF INDIA Indira Paryavaran Bhavan, Jorbagh Road, New Delhi - 110003 Page | 1 Title of Project/Programme: Management of ecosystem of Kaziranga National Park by creating climate resilient livelihood for vulnerable communities through organic farming and pond based pisciculture Project/Programme Objective/s: The proposed project entails the following broad objectives: ► Rejuvenating selected beels which are presently completely dry and doesn’t hold any water, which includes de-siltation of the beel to increase the depth and thus the augment the water holding capacity of the beel. ► Increase in livelihood option for vulnerable communities living in vicinity of Kaziranga National Park through organic farming and pond based fisheries ► Management of watersheds through check dams and ponds Organic farming is envisaged for the vulnerable communities within the southern periphery of the national park. A focused livelihood generation from fisheries is also envisaged for the fishing communities living in the in the north bank of Brahmaputra. Project/ Programme Sector: ► Forestry, agriculture, fisheries and ecosystem Name of Executing Entity/ies/Department: ► Kaziranga National Park (KNP) under Department of Environment & Forests (DoEF), Government of Assam. Beneficiaries: ► Vulnerable communities living in the periphery of Kaziranga National Park (KNP), Assam Project Duration: 3 years Start Date: October 2016 End Date: September 2019 Amount of Financing Requested (INR.): 2,473.08 Lakhs Project Location: The list of finalised project sites are as under. -
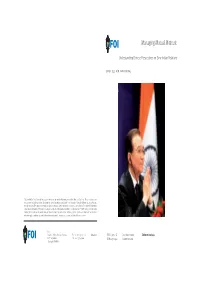
Managing Mutual Mistrust
Managing Mutual Mistrust: Understanding Chinese Perspectives on Sino-Indian Relations JERKER HELLSTRÖM, KAAN KORKMAZ FOI, Swedish Defence Research Agency, is a mainly assignment-funded agency under the Ministry of Defence. The core activities are research, method and technology development, as well as studies conducted in the interests of Swedish defence and the safety and security of society. The organisation employs approximately 1000 personnel of whom about 800 are scientists. This makes FOI Sweden’s largest research institute. FOI gives its customers access to leading-edge expertise in a large number of fi elds such as security policy studies, defence and security related analyses, the assessment of various types of threat, systems for control and management of crises, protection against and management of hazardous substances, IT security and the potential offered by new sensors. FOI Swedish Defence Research Agency Phone: +46 8 555 030 00 www.foi.se FOI-R--3271--SE Base data report Defence Analysis Defence Analysis Fax: +46 8 555 031 00 ISSN 1650-1942 September 2011 SE-164 90 Stockholm Jerker Hellström, Kaan Korkmaz Managing Mutual Mistrust: Understanding Chinese Perspectives on Sino-Indian Relations Cover photo: Chinese Premier Wen Jiabao sits against the backdrop of the Indian flag before delivering a lecture in New Delhi, India, 16 December 2010. (AP Photo/Gurinder Osan) FOI-R--3271--SE Title Managing Mutual Mistrust: Understanding Chinese Perspectives on Sino-Indian Relations Titel Att hantera ömsesidig misstro: kinesiska perspektiv -

Maryland Series in Contemporary Asian Studies Number 3
Maryland Series in Contemporary Asian Studies Number 3 - 2009 0 98) Maryland Series in Contemporary Asian Studies General Editor: Hungdah CHIU Executive Editor: Chih-Yu T. WU Associate Editor: Matthew Lyon Managing Editor: Chih-Yu T. WU Assistant Editor: Timothy A. Costello Jinho SUH Editorial Advisory Board Professor Robert A. Scalapino, University of California at Berkeley Professor Bih-jaw LIN, National Chengchi University Dr. Ying-jeou MA, Chinese Society of International Law Professor Toshio SAWADA, Sophia University, Japan All contributions (in English only) and communications should be sent to: Chih-Yu T. WU University of Maryland School of Law 500 West Baltimore Street Baltimore, Maryland 21201-1786, USA All publications in this series reflect only the views of the authors. While the editor accepts responsibility for the selection of materials to be published, the individual author is responsible for statements of facts and expressions of opinion contained therein. Subscription is US $40.00 per year for 4 issues (regardless of the price of individual issues) in the United States and $45.00 for Canada or overseas. Checks should be addressed to MSCAS. Tel.: (410) 706-3870 Fax: (410) 706-1516 Price for single copy of this issue: US $15.00 ISSN 0730-0107 ISBN 1-932330-28-3 © Maryland Series in Contemporary Asian Studies, Inc. "ALL THAT GLITTERS IS NOT GOLD": TIBET AS A PSEUDO-STATE Barry Sautman* TABLE OF CONTENTS I. INTRODUCTION: THE CONTINUING CLAIM OF TIBETAN STATEHOOD ........................ 3 II. IS INTERNATIONAL LAW RELEVANT TO THE QUESTION OF TIBETAN STATEHOOD? ......... 12 III. "OLD TIBET" AS FIT TO BE INDEPENDENT .. -
Crustal Thickening Prior to 38Ma in Southern Tibet: Evidence From
Gondwana Research 21 (2012) 88–99 Contents lists available at ScienceDirect Gondwana Research journal homepage: www.elsevier.com/locate/gr Crustal thickening prior to 38 Ma in southern Tibet: Evidence from lower crust-derived adakitic magmatism in the Gangdese Batholith Qi Guan a,b, Di-Cheng Zhu a,⁎, Zhi-Dan Zhao a, Guo-Chen Dong a, Liang-Liang Zhang a, Xiao-Wei Li a,c, Min Liu a, Xuan-Xue Mo a, Yong-Sheng Liu d, Hong-Lin Yuan e a State Key Laboratory of Geological Processes and Mineral Resources, and School of Earth Science and Resources, China University of Geosciences, Beijing 100083, China b College of Resources, Shijiazhuang University of Economics, Shijiazhuang 050031, China c School of Earth and Space Sciences, Peking University, Beijing 100871, China d State Key Laboratory of Geological Processes and Mineral Resources, and Faculty of Earth Sciences, China University of Geosciences, Wuhan 430074, China e State Key Laboratory of Continental Dynamics, Department of Geology, Northwest University, Xi'an 710069, China article info abstract Article history: The petrogenesis and geodynamic implications of the Cenozoic adakites in southern Tibet remain topics of debate. Received 17 March 2011 Here we report geochronological and geochemical data for host granites and maficenclavesfromWolonginthe Received in revised form 24 June 2011 eastern Gangdese Batholith, southern Tibet. Zircon LA-ICP-MS dating indicates that the Wolong host granites and Accepted 3 July 2011 enclaves were synchronously emplaced at ca. 38 Ma. The host granites are medium- to high-K calc-alkaline, Available online 14 July 2011 metaluminous (A/CNK=0.93–0.96), with high Al2O3 (15.47–17.68%), low MgO (0.67–1.18%), very low abundances of compatible elements (e.g., Cr=3.87–8.36 ppm, Ni=3.04–5.71 ppm), and high Sr/Y ratios Keywords: – fi – – Zircon U–Pb geochronology (127 217), similar to those typical of adakite. -

And Blue Sheep (Pseudois Nayaur) Populations in the Kangchenjunga Conservation Area (KCA), Nepal
Survey of Snow Leopard (Uncia uncia) and Blue Sheep (Pseudois nayaur) populations in the Kangchenjunga Conservation Area (KCA), Nepal. Final report Submitted to Snow Leopard Trust USA, Seattle By Janak Raj Khatiwada, MSc Principal Investigator Mukesh K. Chalise, PhD, Randall C. Kyes, PhD Participating Investigators February 2007 Executive summary This study was carried out in the Kangchenjunga Conservation Area (KCA), Eastern Nepal from Feb - Nov 2007. We used the Snow Leopard Information Management System, SLIMS (second order survey technique) to determine the relative abundance of snow leopard in the upper part of KCA. Altogether, 36 transects (total length of 15.21 km) were laid down in the major three blocks of KCA. 104 Signs (77 scrapes, 20 feces, 2 Scent mark, 3 Pugmarks and 2 hairs) were recorded. Fixed-point count method was applied for blue sheep from appropriate vantage points. We counted total individual in each herd using 8×42 binocular and 15-60× spotting scope. A total of 43 herds and 1102 individuals were observed in the area. The standard SLIMS questionnaire was conducted to find out relevant information on livestock depredation patterns. Out of 35 households surveyed in KCA, 48% of herders lost livestock due to snow leopards. A total of 21 animals were reportedly lost due to snow leopards from August to September 2007. INTRODUCTION The Snow Leopard (Uncia uncia) is an endangered species lives in the mountains of the Central Asia and the Himalayas, often in very high altitudes with extremely low winter temperatures and far away from sheltering forests (Nowel and Jackson, 1996). In Nepal it inhabits the main Himalayan chain along the Tibetan border (HMG Nepal, 2005).