Final Corrected Binyam Msc Thesis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Districts of Ethiopia
Region District or Woredas Zone Remarks Afar Region Argobba Special Woreda -- Independent district/woredas Afar Region Afambo Zone 1 (Awsi Rasu) Afar Region Asayita Zone 1 (Awsi Rasu) Afar Region Chifra Zone 1 (Awsi Rasu) Afar Region Dubti Zone 1 (Awsi Rasu) Afar Region Elidar Zone 1 (Awsi Rasu) Afar Region Kori Zone 1 (Awsi Rasu) Afar Region Mille Zone 1 (Awsi Rasu) Afar Region Abala Zone 2 (Kilbet Rasu) Afar Region Afdera Zone 2 (Kilbet Rasu) Afar Region Berhale Zone 2 (Kilbet Rasu) Afar Region Dallol Zone 2 (Kilbet Rasu) Afar Region Erebti Zone 2 (Kilbet Rasu) Afar Region Koneba Zone 2 (Kilbet Rasu) Afar Region Megale Zone 2 (Kilbet Rasu) Afar Region Amibara Zone 3 (Gabi Rasu) Afar Region Awash Fentale Zone 3 (Gabi Rasu) Afar Region Bure Mudaytu Zone 3 (Gabi Rasu) Afar Region Dulecha Zone 3 (Gabi Rasu) Afar Region Gewane Zone 3 (Gabi Rasu) Afar Region Aura Zone 4 (Fantena Rasu) Afar Region Ewa Zone 4 (Fantena Rasu) Afar Region Gulina Zone 4 (Fantena Rasu) Afar Region Teru Zone 4 (Fantena Rasu) Afar Region Yalo Zone 4 (Fantena Rasu) Afar Region Dalifage (formerly known as Artuma) Zone 5 (Hari Rasu) Afar Region Dewe Zone 5 (Hari Rasu) Afar Region Hadele Ele (formerly known as Fursi) Zone 5 (Hari Rasu) Afar Region Simurobi Gele'alo Zone 5 (Hari Rasu) Afar Region Telalak Zone 5 (Hari Rasu) Amhara Region Achefer -- Defunct district/woredas Amhara Region Angolalla Terana Asagirt -- Defunct district/woredas Amhara Region Artuma Fursina Jile -- Defunct district/woredas Amhara Region Banja -- Defunct district/woredas Amhara Region Belessa -- -

20210714 Access Snapshot- Tigray Region June 2021 V2
ETHIOPIA Tigray: Humanitarian Access Snapshot (July 2021) As of 31 July 2021 The conflict in Tigray continues despite the unilateral ceasefire announced by the Ethiopian Federal Government on 28 June, which resulted in the withdrawal of the Ethiopian National Overview of reported incidents July Since Nov July Since Nov Defense Forces (ENDF) and Eritrea’s Defense Forces (ErDF) from Tigray. In July, Tigray forces (TF) engaged in a military offensive in boundary areas of Amhara and Afar ERITREA 13 153 2 14 regions, displacing thousands of people and impacting access into the area. #Incidents impacting Aid workers killed Federal authorities announced the mobilization of armed forces from other regions. The Amhara region the security of aid Tahtay North workers Special Forces (ASF), backed by ENDF, maintain control of Western zone, with reports of a military Adiyabo Setit Humera Western build-up on both sides of the Tekezi river. ErDF are reportedly positioned in border areas of Eritrea and in SUDAN Kafta Humera Indasilassie % of incidents by type some kebeles in North-Western and Eastern zones. Thousands of people have been displaced from town Central Eastern these areas into Shire city, North-Western zone. In line with the Access Monitoring and Western Korarit https://bit.ly/3vcab7e May Reporting Framework: Electricity, telecommunications, and banking services continue to be disconnected throughout Tigray, Gaba Wukro Welkait TIGRAY 2% while commercial cargo and flights into the region remain suspended. This is having a major impact on Tselemti Abi Adi town May Tsebri relief operations. Partners are having to scale down operations and reduce movements due to the lack Dansha town town Mekelle AFAR 4% of fuel. -

Technological Gaps of Agricultural Extension: Mismatch Between Demand and Supply in North Gondar Zone, Ethiopia
Vol.10(8), pp. 144-149, August 2018 DOI: 10.5897/JAERD2018.0954 Articles Number: 0B0314557914 ISSN: 2141-2170 Copyright ©2018 Journal of Agricultural Extension and Author(s) retain the copyright of this article http://www.academicjournals.org/JAERD Rural Development Full Length Research Paper Technological gaps of agricultural extension: Mismatch between demand and supply in North Gondar Zone, Ethiopia Genanew Agitew1*, Sisay Yehuala1, Asegid Demissie2 and Abebe Dagnew3 1Department of Rural Development and Agricultural Extension, College of Agriculture and Rural Transformation, University of Gondar, Ethiopia. 2College of Business and Economics, University of Gondar, Ethiopia. 3Departments of Agricultural Economics, College of Agriculture and Rural Transformation, University of Gondar, Ethiopia. Received 27 March, 2018; Accepted 30 April, 2018 This paper examines technological challenges of the agricultural extension in North Gondar Zone of Ethiopia. Understanding technological gaps in public agricultural extension helps to devise demand driven and compatible technologies to existing contexts of farmers. The study used cross sectional survey using quantitative and qualitative techniques. Data were generated from primary and secondary sources using household survey from randomly taken households, focus group discussions, key informant interview, observation and review of relevant documents and empirical works. The result of study shows that there are mismatches between needs of smallholders in crop and livestock production and available agricultural technologies delivered by public agricultural extension system. The existing agricultural technologies are limited and unable to meet the diverse needs of farm households. On the other hand, some of agricultural technologies in place are not appropriate to existing context because of top-down recommendations than need based innovation approaches. -

Phenotypic Characterization of Indigenous Chicken Ecotypes in North Gondar Zone, Ethiopia
Global Veterinaria 12 (3): 361-368, 2014 ISSN 1992-6197 © IDOSI Publications, 2014 DOI: 10.5829/idosi.gv.2014.12.03.76136 Phenotypic Characterization of Indigenous Chicken Ecotypes in North Gondar Zone, Ethiopia 12Addis Getu, Kefyalew Alemayehu and 3Zewdu Wuletaw 1University of Gondar, Faculty of Veterinary Medicine, Department of Animal Production and Extension 2Bahir Dar University College of Agriculture and Environmental Sciences, Department of Animal Production and Technology 3Sustainable Land Management Organization (SLM) Bihar Dar, Ethiopia Abstract: An exploratory field survey was conducted in north Gondar zone, Ethiopia to identify and characterize the local chicken ecotypes. Seven qualitative and twelve quantitative traits from 450 chickens were considered. Chicken ecotypes such as necked neck, Gasgie and Gugut from Quara, Alefa and Tache Armacheho districts were identified, respectively. Morphometric measurements indicated that the body weight and body length of necked neck and Gasgie ecotypes were significantly (p < 0.01) higher than Gugut ecotypes except in shank circumstances. Sex and ecotype were the significant (p < 0.01) sources of variation for both body weights and linear body measurements. The relationship of body weight with other body measurements for all ecotypes in both sexes were highly significant (r = 0.67, p < 0.01). Some traits like wingspan, body length and super length (r = 0.64, P < 0.01) for males and for females (r = 0.59, P < 0.01) of necked neck chickens are significantly correlated with body weight. Therefore, highly correlated traits are the basic indicators for estimation of the continuous prediction of body weight of chicken. Identification and characterization of new genetic resources should be employed routinely to validate and investigate the resources in the country. -

Local History of Ethiopia Ma - Mezzo © Bernhard Lindahl (2008)
Local History of Ethiopia Ma - Mezzo © Bernhard Lindahl (2008) ma, maa (O) why? HES37 Ma 1258'/3813' 2093 m, near Deresge 12/38 [Gz] HES37 Ma Abo (church) 1259'/3812' 2549 m 12/38 [Gz] JEH61 Maabai (plain) 12/40 [WO] HEM61 Maaga (Maago), see Mahago HEU35 Maago 2354 m 12/39 [LM WO] HEU71 Maajeraro (Ma'ajeraro) 1320'/3931' 2345 m, 13/39 [Gz] south of Mekele -- Maale language, an Omotic language spoken in the Bako-Gazer district -- Maale people, living at some distance to the north-west of the Konso HCC.. Maale (area), east of Jinka 05/36 [x] ?? Maana, east of Ankar in the north-west 12/37? [n] JEJ40 Maandita (area) 12/41 [WO] HFF31 Maaquddi, see Meakudi maar (T) honey HFC45 Maar (Amba Maar) 1401'/3706' 1151 m 14/37 [Gz] HEU62 Maara 1314'/3935' 1940 m 13/39 [Gu Gz] JEJ42 Maaru (area) 12/41 [WO] maass..: masara (O) castle, temple JEJ52 Maassarra (area) 12/41 [WO] Ma.., see also Me.. -- Mabaan (Burun), name of a small ethnic group, numbering 3,026 at one census, but about 23 only according to the 1994 census maber (Gurage) monthly Christian gathering where there is an orthodox church HET52 Maber 1312'/3838' 1996 m 13/38 [WO Gz] mabera: mabara (O) religious organization of a group of men or women JEC50 Mabera (area), cf Mebera 11/41 [WO] mabil: mebil (mäbil) (A) food, eatables -- Mabil, Mavil, name of a Mecha Oromo tribe HDR42 Mabil, see Koli, cf Mebel JEP96 Mabra 1330'/4116' 126 m, 13/41 [WO Gz] near the border of Eritrea, cf Mebera HEU91 Macalle, see Mekele JDK54 Macanis, see Makanissa HDM12 Macaniso, see Makaniso HES69 Macanna, see Makanna, and also Mekane Birhan HFF64 Macargot, see Makargot JER02 Macarra, see Makarra HES50 Macatat, see Makatat HDH78 Maccanissa, see Makanisa HDE04 Macchi, se Meki HFF02 Macden, see May Mekden (with sub-post office) macha (O) 1. -

Modeling Malaria Cases Associated with Environmental Risk Factors in Ethiopia Using Geographically Weighted Regression
MODELING MALARIA CASES ASSOCIATED WITH ENVIRONMENTAL RISK FACTORS IN ETHIOPIA USING GEOGRAPHICALLY WEIGHTED REGRESSION Berhanu Berga Dadi i MODELING MALARIA CASES ASSOCIATED WITH ENVIRONMENTAL RISK FACTORS IN ETHIOPIA USING THE GEOGRAPHICALLY WEIGHTED REGRESSION MODEL, 2015-2016 Dissertation supervised by Dr.Jorge Mateu Mahiques,PhD Professor, Department of Mathematics University of Jaume I Castellon, Spain Ana Cristina Costa, PhD Professor, Nova Information Management School University of Nova Lisbon, Portugal Pablo Juan Verdoy, PhD Professor, Department of Mathematics University of Jaume I Castellon, Spain March 2020 ii DECLARATION OF ORIGINALITY I declare that the work described in this document is my own and not from someone else. All the assistance I have received from other people is duly acknowledged, and all the sources (published or not published) referenced. This work has not been previously evaluated or submitted to the University of Jaume I Castellon, Spain, or elsewhere. Castellon, 30th Feburaury 2020 Berhanu Berga Dadi iii Acknowledgments Before and above anything, I want to thank our Lord Jesus Christ, Son of GOD, for his blessing and protection to all of us to live. I want to thank also all consortium of Erasmus Mundus Master's program in Geospatial Technologies for their financial and material support during all period of my study. Grateful acknowledgment expressed to Supervisors: Prof.Dr.Jorge Mateu Mahiques, Universitat Jaume I(UJI), Prof.Dr.Ana Cristina Costa, Universidade NOVA de Lisboa, and Prof.Dr.Pablo Juan Verdoy, Universitat Jaume I(UJI) for their immense support, outstanding guidance, encouragement and helpful comments throughout my thesis work. Finally, but not least, I would like to thank my lovely wife, Workababa Bekele, and beloved daughter Loise Berhanu and son Nethan Berhanu for their patience, inspiration, and understanding during the entire period of my study. -

From Falashas to Ethiopian Jews
FROM FALASHAS TO ETHIOPIAN JEWS: THE EXTERNAL INFLUENCES FOR CHANGE C. 1860-1960 BY DANIEL P. SUMMERFIELD A THESIS SUBMITTED TO THE UNIVERSITY OF LONDON (SCHOOL OF ORIENTAL AND AFRICAN STUDIES) FOR THE DEGREE OF DOCTOR OF PHILOSOPHY (PhD) 1997 ProQuest Number: 10673074 All rights reserved INFORMATION TO ALL USERS The quality of this reproduction is dependent upon the quality of the copy submitted. In the unlikely event that the author did not send a com plete manuscript and there are missing pages, these will be noted. Also, if material had to be removed, a note will indicate the deletion. uest ProQuest 10673074 Published by ProQuest LLC(2017). Copyright of the Dissertation is held by the Author. All rights reserved. This work is protected against unauthorized copying under Title 17, United States C ode Microform Edition © ProQuest LLC. ProQuest LLC. 789 East Eisenhower Parkway P.O. Box 1346 Ann Arbor, Ml 48106- 1346 ABSTRACT The arrival of a Protestant mission in Ethiopia during the 1850s marks a turning point in the history of the Falashas. Up until this point, they lived relatively isolated in the country, unaffected and unaware of the existence of world Jewry. Following this period and especially from the beginning of the twentieth century, the attention of certain Jewish individuals and organisations was drawn to the Falashas. This contact initiated a period of external interference which would ultimately transform the Falashas, an Ethiopian phenomenon, into Ethiopian Jews, whose culture, religion and identity became increasingly connected with that of world Jewry. It is the purpose of this thesis to examine the external influences that implemented and continued the process of transformation in Falasha society which culminated in their eventual emigration to Israel. -

Ethiopia Final Evaluation Report
ETHIOPIA: Mid-Term Evaluation of UNCDF’s Local Development Programme Submitted to: United Nations Capital Development Fund Final Evaluation Report 23 July 2007 Prepared by: Maple Place North Woodmead Business Park 145 Western Service Road Woodmead 2148 Tel: +2711 802 0015 Fax: +2711 802 1060 www.eciafrica.com UNITED NATIONS DEVELOPMENT FUND EVALUATION REPORT TABLE OF CONTENTS 1. PROJECT SUMMARY 1 2. PURPOSE OF THE EVALUATION 2 Purpose of the evaluation 2 Programme Cycle 2 3. EVALUATION METHODOLOGY 3 Methodology and tools used 3 Work plan 4 Team composition 5 4. PROGRAMME PROFILE 6 Understanding the context 6 Donor Interventions in Amhara Region 7 Programme Summary 8 Programme Status 9 5. KEY EVALUATION FINDINGS 13 Results achievement 13 Sustainability of results 21 Factors affecting successful implementation & results achievement 24 External Factors 24 Programme related factors 24 Strategic position and partnerships 26 Future UNCDF role 27 6. LESSONS 28 Programme-level lessons 28 7. RECOMMENDATIONS 29 Results achievement 29 Sustainability of results 29 Factors affecting successful implementation and results achievement 30 Strategic positioning and partnerships 31 Future UNCDF role 31 “The analysis and recommendations of this report do not necessarily reflect the view of the United Nations Capital Development Fund, its Executive Board or the United Nations Member States. This is an independent publication of UNCDF and reflects the views of its authors” PREPARED BY ECIAFRICA CONSULTING (PTY) LTD, PROPRIETARY AND CONFIDENTIAL i 2007/05/24 -
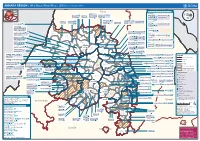
AMHARA REGION : Who Does What Where (3W) (As of 13 February 2013)
AMHARA REGION : Who Does What Where (3W) (as of 13 February 2013) Tigray Tigray Interventions/Projects at Woreda Level Afar Amhara ERCS: Lay Gayint: Beneshangul Gumu / Dire Dawa Plan Int.: Addis Ababa Hareri Save the fk Save the Save the df d/k/ CARE:f k Save the Children:f Gambela Save the Oromia Children: Children:f Children: Somali FHI: Welthungerhilfe: SNNPR j j Children:l lf/k / Oxfam GB:af ACF: ACF: Save the Save the af/k af/k Save the df Save the Save the Tach Gayint: Children:f Children: Children:fj Children:l Children: l FHI:l/k MSF Holand:f/ ! kj CARE: k Save the Children:f ! FHI:lf/k Oxfam GB: a Tselemt Save the Childrenf: j Addi Dessie Zuria: WVE: Arekay dlfk Tsegede ! Beyeda Concern:î l/ Mirab ! Concern:/ Welthungerhilfe:k Save the Children: Armacho f/k Debark Save the Children:fj Kelela: Welthungerhilfe: ! / Tach Abergele CRS: ak Save the Children:fj ! Armacho ! FHI: Save the l/k Save thef Dabat Janamora Legambo: Children:dfkj Children: ! Plan Int.:d/ j WVE: Concern: GOAL: Save the Children: dlfk Sahla k/ a / f ! ! Save the ! Lay Metema North Ziquala Children:fkj Armacho Wegera ACF: Save the Children: Tenta: ! k f Gonder ! Wag WVE: Plan Int.: / Concern: Save the dlfk Himra d k/ a WVE: ! Children: f Sekota GOAL: dlf Save the Children: Concern: Save the / ! Save: f/k Chilga ! a/ j East Children:f West ! Belesa FHI:l Save the Children:/ /k ! Gonder Belesa Dehana ! CRS: Welthungerhilfe:/ Dembia Zuria ! î Save thedf Gaz GOAL: Children: Quara ! / j CARE: WVE: Gibla ! l ! Save the Children: Welthungerhilfe: k d k/ Takusa dlfj k -

Transhumance Cattle Production System in North Gondar, Amhara Region, Ethiopia: Is It Sustainable?
WP14_Cover.pdf 2/12/2009 2:21:51 PM www.ipms-ethiopia.org Working Paper No. 14 Transhumance cattle production system in North Gondar, Amhara Region, Ethiopia: Is it sustainable? C M Y CM MY CY CMY K Transhumance cattle production system in North Gondar, Amhara Region, Ethiopia: Is it sustainable? Azage Tegegne,* Tesfaye Mengistie, Tesfaye Desalew, Worku Teka and Eshete Dejen Improving Productivity and Market Success (IPMS) of Ethiopian Farmers Project, International Livestock Research Institute (ILRI), Addis Ababa, Ethiopia * Corresponding author: [email protected] Authors’ affiliations Azage Tegegne, Improving Productivity and Market Success (IPMS) of Ethiopian Farmers Project, International Livestock Research Institute (ILRI), Addis Ababa, Ethiopia Tesfaye Mengistie, Bureau of Agriculture and Rural Development, Amhara Regional State, Ethiopia Tesfaye Desalew, Kutaber woreda Office of Agriculture and Rural Development, Kutaber, South Wello Zone, Amhara Regional State, Ethiopia Worku Teka, Research and Development Officer, Metema, Amhara Region, Improving Productivity and Market Success (IPMS) of Ethiopian Farmers Project, International Livestock Research Institute (ILRI), Addis Ababa, Ethiopia Eshete Dejen, Amhara Regional Agricultural Research Institute (ARARI), P.O. Box 527, Bahir Dar, Amhara Regional State, Ethiopia © 2009 ILRI (International Livestock Research Institute). All rights reserved. Parts of this publication may be reproduced for non-commercial use provided that such reproduction shall be subject to acknowledgement of ILRI as holder of copyright. Editing, design and layout—ILRI Publications Unit, Addis Ababa, Ethiopia. Correct citation: Azage Tegegne, Tesfaye Mengistie, Tesfaye Desalew, Worku Teka and Eshete Dejen. 2009. Transhumance cattle production system in North Gondar, Amhara Region, Ethiopia: Is it sustainable? IPMS (Improving Productivity and Market Success) of Ethiopian Farmers Project. -

Prospección Arqueológica Y Etnoarqueológica De Metema Y Qwara (Etiopía) 95 Prospección Arqueológica Y Etnoarqueológica De Metema Y Qwara (Etiopía)
Prospección arqueológica y etnoarqueológica de Metema y Qwara (Etiopía) 95 Prospección arqueológica y etnoarqueológica de Metema y Qwara (Etiopía) Alfredo González-Ruibal Incipit-CSIC [email protected] Xurxo Ayán Vila GPAC-Universidad del País Vasco [email protected] Worku Derara Megenassa Department of Archaeology, Addis Ababa University [email protected] Álvaro Falquina Aparicio Arqueólogo [email protected] Manuel Sánchez-Elipe Lorente Departamento de Prehistoria, Universidad Complutense de Madrid [email protected] Resumen: el área noroccidental de Etiopía es desconocida desde un punto de vista arqueológi- co, pese a su importancia como una zona de transición entre las tierras bajas sudanesas y el altiplano etíope. En 2013 llevamos a cabo la primera prospección arqueológica y etnoarqueoló- gica del área. Durante nuestra investigación descubrimos tanto yacimientos prehistóricos como históricos. Por lo que se refiere a los primeros, se trata de áreas de dispersión de materiales líti- cos pertenecientes mayoritariamente a la Late Stone Age. Más relevante es el descubrimiento de un abrigo rocoso con pinturas esquemáticas, dada su rareza en la frontera etíope-sudanesa. La mayor parte de los yacimientos históricos son fuertes de piedra datados en la segunda mitad del siglo XIX, la mayor parte de los cuales se reocuparon durante el periodo italiano (1936-1941). Desde el punto de vista de la etnoarqueología destaca el «descubrimiento» de un grupo étnico no documentado: los dats’in, hablantes de una lengua desconocida emparentada con el gumuz. Palabras clave: Late Stone Age (LSA), arte rupestre, Sudán turco-egipcio, colonialismo italiano, fuertes fronterizos, pueblos nilo-saharianos. Informes y trabajos 12 | Págs. -

Ethiopia Round 6 SDP Questionnaire
Ethiopia Round 6 SDP Questionnaire Always 001a. Your name: [NAME] Is this your name? ◯ Yes ◯ No 001b. Enter your name below. 001a = 0 Please record your name 002a = 0 Day: 002b. Record the correct date and time. Month: Year: ◯ TIGRAY ◯ AFAR ◯ AMHARA ◯ OROMIYA ◯ SOMALIE BENISHANGUL GUMZ 003a. Region ◯ ◯ S.N.N.P ◯ GAMBELA ◯ HARARI ◯ ADDIS ABABA ◯ DIRE DAWA filter_list=${this_country} ◯ NORTH WEST TIGRAY ◯ CENTRAL TIGRAY ◯ EASTERN TIGRAY ◯ SOUTHERN TIGRAY ◯ WESTERN TIGRAY ◯ MEKELE TOWN SPECIAL ◯ ZONE 1 ◯ ZONE 2 ◯ ZONE 3 ZONE 5 003b. Zone ◯ ◯ NORTH GONDAR ◯ SOUTH GONDAR ◯ NORTH WELLO ◯ SOUTH WELLO ◯ NORTH SHEWA ◯ EAST GOJAM ◯ WEST GOJAM ◯ WAG HIMRA ◯ AWI ◯ OROMIYA 1 ◯ BAHIR DAR SPECIAL ◯ WEST WELLEGA ◯ EAST WELLEGA ◯ ILU ABA BORA ◯ JIMMA ◯ WEST SHEWA ◯ NORTH SHEWA ◯ EAST SHEWA ◯ ARSI ◯ WEST HARARGE ◯ EAST HARARGE ◯ BALE ◯ SOUTH WEST SHEWA ◯ GUJI ◯ ADAMA SPECIAL ◯ WEST ARSI ◯ KELEM WELLEGA ◯ HORO GUDRU WELLEGA ◯ Shinile ◯ Jijiga ◯ Liben ◯ METEKEL ◯ ASOSA ◯ PAWE SPECIAL ◯ GURAGE ◯ HADIYA ◯ KEMBATA TIBARO ◯ SIDAMA ◯ GEDEO ◯ WOLAYITA ◯ SOUTH OMO ◯ SHEKA ◯ KEFA ◯ GAMO GOFA ◯ BENCH MAJI ◯ AMARO SPECIAL ◯ DAWURO ◯ SILTIE ◯ ALABA SPECIAL ◯ HAWASSA CITY ADMINISTRATION ◯ AGNEWAK ◯ MEJENGER ◯ HARARI ◯ AKAKI KALITY ◯ NEFAS SILK-LAFTO ◯ KOLFE KERANIYO 2 ◯ GULELE ◯ LIDETA ◯ KIRKOS-SUB CITY ◯ ARADA ◯ ADDIS KETEMA ◯ YEKA ◯ BOLE ◯ DIRE DAWA filter_list=${level1} ◯ TAHTAY ADIYABO ◯ MEDEBAY ZANA ◯ TSELEMTI ◯ SHIRE ENIDASILASE/TOWN/ ◯ AHIFEROM ◯ ADWA ◯ TAHTAY MAYCHEW ◯ NADER ADET ◯ DEGUA TEMBEN ◯ ABIYI ADI/TOWN/ ◯ ADWA/TOWN/ ◯ AXUM/TOWN/ ◯ SAESI TSADAMBA ◯ KLITE