Original Research Original Research
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Addis Ababa University
ADDIS ABABA UNIVERSITY COLLEGE OF EDUCATION AND BEHAVIORAL SCIENCES DEPARTMENT OF EDUCATIONAL PLANNING AND MANAGEMENT Thesis Report Challenges of Parental Involvement in Students' Learning At Boneya Boshe Woreda of East Wollaga Zone By Sekata Negewo Advisor: Getachew Adugna (PhD) June,2018 Addis Ababa CHALLENGES OF PARENTS’ INVOLVEMENT IN STUDENTS’ LEARNING AT BONEYA BOSHE DISTRICT OF EAST WOLLEGA ZONE By: Sekata Negewo Gobena Advisor: Getachew Adugna (PhD) A THESIS SUBMITTED TO THE DEPARTMENT OF EDUCATIONAL PLANNING AND MANAGEMENT IN PARTIAL FULFILLMENT FOR THE REQUIREMENTS OF MASTER OF ARTS IN SCHOOL LEADERSHIP JUNE, 2018 ADDIS ABABA UNIVERSITY Declaration The researcher here by declares that the thesis on the title; ―practices and challenges of parents‘ involvement in student‘s academic achievement: the case of Boneya Boshe Woreda Late Cycle Primary Schools.‘‘, is his original work and that all sources that have been referred to and quoted have been dully indicated and acknowledged with complete references. Name- Sekata Negewo Sign-__________________ Date- __________________ This thesis has been submitted for examination with my approval as the university advisor. Main advisor- Name -Getachew Adugna (Ph. D) Sign __________________ Date ___________________ Co- advisor- Name ______________ Sign _________________________ Date ________________________ Place: Addis Ababa University College of Education and Behavioral Science Department of Educational Planning and Management. Date of submission ___________________ ADDIS ABABA UNIVERSITY -

Districts of Ethiopia
Region District or Woredas Zone Remarks Afar Region Argobba Special Woreda -- Independent district/woredas Afar Region Afambo Zone 1 (Awsi Rasu) Afar Region Asayita Zone 1 (Awsi Rasu) Afar Region Chifra Zone 1 (Awsi Rasu) Afar Region Dubti Zone 1 (Awsi Rasu) Afar Region Elidar Zone 1 (Awsi Rasu) Afar Region Kori Zone 1 (Awsi Rasu) Afar Region Mille Zone 1 (Awsi Rasu) Afar Region Abala Zone 2 (Kilbet Rasu) Afar Region Afdera Zone 2 (Kilbet Rasu) Afar Region Berhale Zone 2 (Kilbet Rasu) Afar Region Dallol Zone 2 (Kilbet Rasu) Afar Region Erebti Zone 2 (Kilbet Rasu) Afar Region Koneba Zone 2 (Kilbet Rasu) Afar Region Megale Zone 2 (Kilbet Rasu) Afar Region Amibara Zone 3 (Gabi Rasu) Afar Region Awash Fentale Zone 3 (Gabi Rasu) Afar Region Bure Mudaytu Zone 3 (Gabi Rasu) Afar Region Dulecha Zone 3 (Gabi Rasu) Afar Region Gewane Zone 3 (Gabi Rasu) Afar Region Aura Zone 4 (Fantena Rasu) Afar Region Ewa Zone 4 (Fantena Rasu) Afar Region Gulina Zone 4 (Fantena Rasu) Afar Region Teru Zone 4 (Fantena Rasu) Afar Region Yalo Zone 4 (Fantena Rasu) Afar Region Dalifage (formerly known as Artuma) Zone 5 (Hari Rasu) Afar Region Dewe Zone 5 (Hari Rasu) Afar Region Hadele Ele (formerly known as Fursi) Zone 5 (Hari Rasu) Afar Region Simurobi Gele'alo Zone 5 (Hari Rasu) Afar Region Telalak Zone 5 (Hari Rasu) Amhara Region Achefer -- Defunct district/woredas Amhara Region Angolalla Terana Asagirt -- Defunct district/woredas Amhara Region Artuma Fursina Jile -- Defunct district/woredas Amhara Region Banja -- Defunct district/woredas Amhara Region Belessa -- -
Administrative Region, Zone and Woreda Map of Oromia a M Tigray a Afar M H U Amhara a Uz N M
35°0'0"E 40°0'0"E Administrative Region, Zone and Woreda Map of Oromia A m Tigray A Afar m h u Amhara a uz N m Dera u N u u G " / m r B u l t Dire Dawa " r a e 0 g G n Hareri 0 ' r u u Addis Ababa ' n i H a 0 Gambela m s Somali 0 ° b a K Oromia Ü a I ° o A Hidabu 0 u Wara o r a n SNNPR 0 h a b s o a 1 u r Abote r z 1 d Jarte a Jarso a b s a b i m J i i L i b K Jardega e r L S u G i g n o G A a e m e r b r a u / K e t m uyu D b e n i u l u o Abay B M G i Ginde e a r n L e o e D l o Chomen e M K Beret a a Abe r s Chinaksen B H e t h Yaya Abichuna Gne'a r a c Nejo Dongoro t u Kombolcha a o Gulele R W Gudetu Kondole b Jimma Genete ru J u Adda a a Boji Dirmeji a d o Jida Goro Gutu i Jarso t Gu J o Kembibit b a g B d e Berga l Kersa Bila Seyo e i l t S d D e a i l u u r b Gursum G i e M Haro Maya B b u B o Boji Chekorsa a l d Lalo Asabi g Jimma Rare Mida M Aleltu a D G e e i o u e u Kurfa Chele t r i r Mieso m s Kegn r Gobu Seyo Ifata A f o F a S Ayira Guliso e Tulo b u S e G j a e i S n Gawo Kebe h i a r a Bako F o d G a l e i r y E l i Ambo i Chiro Zuria r Wayu e e e i l d Gaji Tibe d lm a a s Diga e Toke n Jimma Horo Zuria s e Dale Wabera n a w Tuka B Haru h e N Gimbichu t Kutaye e Yubdo W B Chwaka C a Goba Koricha a Leka a Gidami Boneya Boshe D M A Dale Sadi l Gemechis J I e Sayo Nole Dulecha lu k Nole Kaba i Tikur Alem o l D Lalo Kile Wama Hagalo o b r Yama Logi Welel Akaki a a a Enchini i Dawo ' b Meko n Gena e U Anchar a Midega Tola h a G Dabo a t t M Babile o Jimma Nunu c W e H l d m i K S i s a Kersana o f Hana Arjo D n Becho A o t -

Ethiopia: Administrative Map (August 2017)
Ethiopia: Administrative map (August 2017) ERITREA National capital P Erob Tahtay Adiyabo Regional capital Gulomekeda Laelay Adiyabo Mereb Leke Ahferom Red Sea Humera Adigrat ! ! Dalul ! Adwa Ganta Afeshum Aksum Saesie Tsaedaemba Shire Indasilase ! Zonal Capital ! North West TigrayTahtay KoraroTahtay Maychew Eastern Tigray Kafta Humera Laelay Maychew Werei Leke TIGRAY Asgede Tsimbila Central Tigray Hawzen Medebay Zana Koneba Naeder Adet Berahile Region boundary Atsbi Wenberta Western Tigray Kelete Awelallo Welkait Kola Temben Tselemti Degua Temben Mekele Zone boundary Tanqua Abergele P Zone 2 (Kilbet Rasu) Tsegede Tselemt Mekele Town Special Enderta Afdera Addi Arekay South East Ab Ala Tsegede Mirab Armacho Beyeda Woreda boundary Debark Erebti SUDAN Hintalo Wejirat Saharti Samre Tach Armacho Abergele Sanja ! Dabat Janamora Megale Bidu Alaje Sahla Addis Ababa Ziquala Maychew ! Wegera Metema Lay Armacho Wag Himra Endamehoni Raya Azebo North Gondar Gonder ! Sekota Teru Afar Chilga Southern Tigray Gonder City Adm. Yalo East Belesa Ofla West Belesa Kurri Dehana Dembia Gonder Zuria Alamata Gaz Gibla Zone 4 (Fantana Rasu ) Elidar Amhara Gelegu Quara ! Takusa Ebenat Gulina Bugna Awra Libo Kemkem Kobo Gidan Lasta Benishangul Gumuz North Wello AFAR Alfa Zone 1(Awsi Rasu) Debre Tabor Ewa ! Fogera Farta Lay Gayint Semera Meket Guba Lafto DPubti DJIBOUTI Jawi South Gondar Dire Dawa Semen Achefer East Esite Chifra Bahir Dar Wadla Delanta Habru Asayita P Tach Gayint ! Bahir Dar City Adm. Aysaita Guba AMHARA Dera Ambasel Debub Achefer Bahirdar Zuria Dawunt Worebabu Gambela Dangura West Esite Gulf of Aden Mecha Adaa'r Mile Pawe Special Simada Thehulederie Kutaber Dangila Yilmana Densa Afambo Mekdela Tenta Awi Dessie Bati Hulet Ej Enese ! Hareri Sayint Dessie City Adm. -

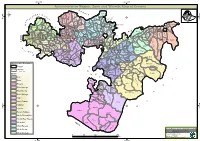
OROMIA REGION - Regional 3W Map 07 December 2010
OROMIA REGION - Regional 3W Map 07 December 2010 CRS I SC-UK V Legend W Amhara S d S Farm Africa R S i E R C Benishangul R A V E CRS CARE MfM C GOAL C P n R W S o i I International Boundary SC-Denmark A , t Gumuz Afar C L c C P K Action Aid C A CARE Welthungerhilfe A CRS S U I M - I ! O C C IMC S S G S a CARE A WVE S Regional Boundary , SC-Denmark R R c m i SC-Denmark Dera C C a S r R f u u u c i C U E A Action Aid t r S m r n u f GOAL e R m R a r A m E IMC r i E C A b a I L L a R R Zonal Boundary K CARE ! f F o C Action Aid H s A a Hidabu Abote m r ! A A g a A r J x a a e A a C r Christian Aid C O d a C d r m O a r b O IMC Action Aid i W a M CA RE o J e I a G F G G G u e ! g L t n b r b e i a i i n i J o E S m u D a d Farm Africa Gerar Jarso R CARE Woreda Boundary E IMC u e p Kuyu E a ! Kiltu Kara m A i n ! o R ! S R C r ! a L Abay Chomen B Debre Libanos o ! Abuna G/Beret A M m M e A H e l o C en a m ks e ina r u Yaya Gulele Abichuna Gne'a C Ch Abe Dongoro ! a h s ! e i t c r a g f a u Nejo t IMC a ! r CRS No Data /No Intervention g E J ! W ! l a x Kombolcha o e R o ! d V e O b B i o ! ! s Guduru G J ib it Goro Gutu ! a r m b a Gudetu Kondole FHI Ke W a Mercy Corps b a s B u i ! r B Boji Dirmeji o t a Bila Seyo e i ! a r Jimma Genete l J d K L e g t s l Jeldu u E a ! d D ! u F Haro Maya e Guto Gida b M S u A m A o e B e M l R i d ! Boji Chekorsa Jimma Rare S ! D CARE GOAL CRS b a B b u A o Aleltu l e ! Kurfa Chele A g I O u f e e y G i u a S Mieso r Agriculture & Livestock i C r s t ! T a Mida KegnA a M Lalo Asabi im r K a ! ! G S Gobu -

Operational Dashboard SMS East Wellega May 2019
SITE MANAGEMENT SMS Operational Dashboard SUPPORT East Wellega (May 2019) Publication: 02 May 2019 DRAFT ETHIOPIA Overview Following the inter-communal conflict that erupted in late September 2018 around border areas of East and Operational presence of cluster partners (East Wellega) West Wollega Zones of Oromia Region and Kemashi Zone Benishangul-Gumz Region caused displacement of hundreds of thousands from both regions. As the situation remained tense and unsettled, IOM started Metekel life-saving interventions including SMS activities late January 2019. IOM SMS Programme is currently operate in 8 collective sites in East Wellega. The total number of IDPs reached by SMS is 12,256, with 2,097 household. Currently, SMS has deployed 8 staff on duty travel to support the IDP crisis in East Wellega Zone, coordinating with over 10 partners across the zone, both local and International NGOs, and UN agencies. Ibantu Key Figures Kiremu 12,256 2,097 8 8 Total Number of IDPs Total Number of IDPs Total Number of Collective Total Number of SMS Staff Supported by SMS deployed to support the Ops HH Supported by SMS Sites Supported by SMS Gida Ayana Horo Guduru Demographics Vulnerabilities Haro Limu 0% Unaccompanied children East Wellega 1 Male Female Haro Limu Limu (Oromia) 0% Orphaned children IOM, WFP, Gov’t, East Wellega 1 52% Individuals 48% 666 Guto Gida GOAL, ICRC, OCHA, 8% Pregnant and lactating Women IOM, Gov’t, WVI, 12% 0 - 5 years 11% IRC, SCI, FIDO, WVI. OCHA, FIDO, SCI 2% Elderly people 19% 6 -17 years 18% 20% 18-59 years 18% 0% People -

ETHIOPIA IDP Situation Report May 2019
ETHIOPIA IDP Situation Report May 2019 Highlights • Government return operations continue at full scale and sites are being dismantled. • Where security is assured and rehabilitation support provided, IDPs have opted to return to their areas of origin. IDPs who still feel insecure and have experienced trauma prefer to relocate elsewhere or integrate within the community. Management of IDP preferences differs in every IDP caseload. • There is minimal to no assistance in areas of return. Local authorities have requested international partner sup- port to address the gap. Meanwhile, public-private initiatives continue to fundraise for the rehabilitation of IDPs. • The living condition of the already vulnerable host communities has deteriorated having shared their limited resources with the IDPs for over a year. I. Displacement context Government IDP return operations have been implemented at full scale since early May 2019 following the 8 April 2019 announcement of the Federal Government’s Strategic Plan to Address Internal Displacement and a costed Re- covery/Rehabilitation Plan. By end May, most IDP sites/camps were dismantled, in particular in East/West Wollega and Gedeo/West Guji zones. Humanitarian partners have increased their engagement with Government at all levels aiming to improve the implementation of the Government return operation, in particular advocating for the returns to happen voluntarily, in safety, sustainably and with dignity. Overall, humanitarian needs remain high in both areas of displacement and of return. Most assistance in displace- ment areas is disrupted following the mass Government return operation and the dismantling of sites, while assis- tance in areas of return remain scant to non-existent, affecting the sustainability of the returns. -

31052019 West & East Wellega & Kemashi Assosa (BGR
ETHIOPIA East &West Wellega zone(Oromia Region)and Kamashi zone(BGR) IDP Returnees Snapshot As of 31 May 2019 Following the inter-communal conflict Awi that erupted in Kamashi zone (BGR) on West Gojam 26 September, 2018 there was massive displacement of people (majority being ± women and children) triggering protec- Metekel tion concerns. KEY FIGURES DISPLACEMENT Sherkole Kurmuk number of IDPs were living in collective 228,954 Sodal (36,320 HH) sites in East & West Wellega, 145, 685 IDPs were living in East Wellega and 83,269 IDPs 9,802 Homosha were living in West Wellega (Source: Menge DRMO) 14,473 Asosa Oda Bilidigilu 354 IDP Returnees Ibantu 26,710 Kiremu In East Wollega, 89,265 IDPs have returned to their place of origin Assosa in East Wellega zone along border areas of Oromia region. 26,985 Yaso 30,468 East Wellega Gida Ayana IDPs have returned to their place of origin in Yaso and Belo Jegan- 4,480 2,592 foy woredas of Kamashi Zone (BGR) from East Wellega Zone. Over- Bambasi Mendi Town Agalometi Haro Limu all,80% of the IDPs from East Wollega have returned to their place Mana Sibu of origin along the border of Oromia region and Kamashi zone Kiltu Kara 14,020 Limu (Oromia) Kemashi (BGR). 20% of the IDPs are living within the host community. Leta Sibu Nejo 12,744 5,986 In West Wollega, 50, 555 IDPs have returned to their place of origin 5,187 Maokomo Special West Wellega Nejo Town Kamashi in Oda Bildigilu Woreda of Assosa Zone and Sedal, Agelo Meti and 9,743 145,685 Indv. -

Adoption of Coffee Shade Agroforestry Technology and Shade Tree
Hindawi Advances in Agriculture Volume 2021, Article ID 8574214, 13 pages https://doi.org/10.1155/2021/8574214 Research Article Adoptionof CoffeeShadeAgroforestry TechnologyandShadeTree Management in Gobu Seyo District, East Wollega, Oromia Tolera Urgessa Waktola 1 and Kidist Fekadu 2 1Self Help Africa Ethiopian Office, Addis Ababa, Ethiopia 2Hawassa University Wondo Genet College of Forestry and Natural Resources, Wondo Genet, Ethiopia Correspondence should be addressed to Kidist Fekadu; [email protected] Received 21 May 2019; Revised 17 March 2021; Accepted 4 June 2021; Published 8 July 2021 Academic Editor: Volkan Okatan Copyright © 2021 Tolera Urgessa Waktola and Kidist Fekadu. .is is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Coffee production in the form of agroforestry practices is the most important management approach to improve the livelihoods of the farming community. .is study was conducted to assess factors affecting the adoption of the technology, its socioeconomic and environmental benefits, and the management practices related to the technology. Out of eight kebeles in the district, two kebeles were selected purposively based on the existing and extensive agroforestry practices. Four villages were selected randomly. Based on the preidentified criteria as coffee growers and nongrowers, coffee growers were selected purposively. .en a total of 120 households were selected by Simple Random Sampling Method. Data were collected by using structured interview and field observation. .e data were analyzed by using descriptive statistics analysis. .e logit model was used to identify the factors affecting the adoption of coffee shade agroforestry technology. -

Trend Analysis of Malaria Prevalence in East Wollega Zone, Oromia Regional State, Western Ethiopia, 2020: a Retrospective Study
omens H OPEN ACCESS Freely available online f W ea o l l th a n C r a u r e o J Journal of Women’s Health Care ISSN: 2167-0420 Research Article Trend Analysis of Malaria Prevalence in East Wollega Zone, Oromia Regional State, Western Ethiopia, 2020: A retrospective study. Zalalem Kaba Babure1*, Yusuf Mohammed Ahmed2, Solomon Tefera Likasa3, Fekadu Assefa Jiru4, Tesfaye Dagne Weldemarium5, Meseret Belete Fite6 1Public Health Specialist at East Wollega Zonal Health Office, Nekemte Town, Oromia Regional State, Western Ethiopia; 2WHE Public Health Emergency Surveillance Officer, East Wollega, Nekemte, Ethiopia; 3East Wollega Zonal Health Office, Nekemte Town, Oromia Regional State, Western Ethiopia; 4Technical Advisor at Oromia Regional Health Bureau, Addis Abeba, Ethiopia; 5St. Paul’s Millennium Medical College, Addis Abeba, Ethiopia; 6Wollega University, Nekemte Town, Western Ethiopia ABSTRACT Background: Malaria, a common and life-threatening disease in many tropical and subtropical areas, caused by infection of red blood cells with protozoan parasites of the genus plasmodium inoculated into the human host by a feeding female anopheline mosquito. Malaria is a major public health problem in Ethiopia and has been consistently reported as one of the three leading top causes of morbidity and mortality. In East Wollega Zone there is lack of empirical evidences on the level of malaria prevalence. Material and Methods: A retrospective study was carried out to determine the two year (July 2018 to June 2020) malaria prevalence based on district health information system version two (dhis2) database reports. All malaria cases reported in the specified periods were carefully reviewed by using questionnaire and analysed. -

Addis Ababa University
ADDIS ABABA UNIVERSITY SCHOOL OF GRADUATE STUDIES COLLEGE OF DEVELOPMENT STUDIES TOURISM DEVELOPMENT AND MANAGEMENT PROGRAM COLLABORATION OF STAKEHOLDERS’ OPERATION FOR TOURISM DEVELOPMENT: EVIDENCES FROM NEKEMTE TOWN AS A TOURIST DESTINATION IN WESTERN OROMIA, ETHIOPIA A THESIS SUBMITTED TO ADDIS ABABA UNIVERSITY, CENTER FOR ENVIRONMENT AND DEVELOPMENT STUDIES, TOURISM DEVELOPMENTAND MANAGEMENT PROGRAM IN PARTIAL FULFILLMENT OF THE REQUIREMENT FOR MASTERS OF ARTS IN TOURISM DEVELOPMENT AND MANAGEMENT BY: ETEFA GUDINA ADVISOR: TESFAYE ZELEKE (PhD) ADDIS ABABA UNIVERSITY ADDIS ABABA, ETHIOPIA JUNE, 2018 COLLABORATION OF STAKEHOLDERS’ OPERATION FOR TOURISM DEVELOPMENT: EVIDENCES FROM NEKEMTE TOWN AS A TOURIST DESTINATION IN WESTERN OROMIA, ETHIOPIA ETEFA GUDINA KITESSA Approved by: _____________________ __________ _________ Thesis Advisor Signature Date ___________________ __________ _________ Internal Examiner Signature Date ______________________ __________ _________ External Examiner Signature Date _____________________ __________ _________ Chairman, Department Signature Date ii Table of Contents Table of Contents ...................................................................................................................... iii DECLARATION ..................................................................................................................... vii DEDICATION ........................................................................................................................ viii List of Tables ........................................................................................................................... -

Ethiopia End of Spray Operations Report 2012
PMI | Africa IRS (AIRS) Project Indoor Residual Spraying (IRS 2) Task Order Four ETHIOPIA END OF SPRAY OPERATIONS REPORT 2012 Contract GHN-I-00-09-00013-00 Task Order: AID-OAA-TO-11-00039 Submitted to: United States Agency for International Development/PMI Submission One on: October 3, 2012 Resubmission on: November 30, 2012 Approved on: February 25, 2013 Prepared by: Abt Associates Inc. Abt Associates Inc. I 4550 Montgomery Avenue I Suite 800 North I Bethesda, Maryland 20814 I T. 301.347.5000 I F. 301.913.9061 I www.abtassociates.com ii ETHIOPIA END OF SPRAY OPERATIONS REPORT 2012 The views expressed in this document do not necessarily reflect the views of the United States Agency for International Development or the United States Government. Contents Acronyms .................................................................................................................................... 5 Executive Summary ...................................................................................................................... 6 1. Introduction ............................................................................................................................ 9 1.1 Project Objectives in 2012 ............................................................................................................................... 9 1.2 Spray Sites ....................................................................................................................................................... 10 1.3 Insecticides ......................................................................................................................................................