Diversity Partitioning During the Cambrian Radiation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Early Paleozoic Life & Extinctions (Part 1)
NJU Course Extinctions: Past, Present & Future Prof. Norman MacLeod School of Earth Sciences & Engineering, Nanjing University Extinctions: Past, Present & Future Extinctions: Past, Present & Future Course Syllabus (Revised) Section Week Title Introduction 1 Course Introduction, Intro. To Extinction Introduction 2 History of Extinction Studies Introduction 3 Evolution, Fossils, Time & Extinction Precambrian Extinctions 4 Origin of Life & Precambrian Extionctions Paleozoic Extinctions 5 Early Paleozoic World & Extinctions Paleozoic Extinctions 6 Middle Paleozoic World & Extinctions Paleozoic Extinctions 7 Late Paleozoic World & Extinctions Assessment 8 Mid-Term Examination Mesozoic Extinctions 9 Triassic-Jurassic World & Extinctions Mesozoic Extinctions 10 Labor Day Holiday Cenozoic Extinctions 11 Cretaceous World & Extinctions Cenozoic Extinctions 12 Paleogene World & Extinctions Cenozoic Extinctions 13 Neogene World & Extinctions Modern Extinctions 14 Quaternary World & Extinctions Modern Extinctions 15 Modern World: Floras, Faunas & Environment Modern Extinctions 16 Modern World: Habitats & Organisms Assessment 17 Final Examination Early Paleozoic World, Life & Extinctions Norman MacLeod School of Earth Sciences & Engineering, Nanjing University Early Paleozoic World, Life & Extinctions Objectives Understand the structure of the early Paleozoic world in terms of timescales, geography, environ- ments, and organisms. Understand the structure of early Paleozoic extinction events. Understand the major Paleozoic extinction drivers. Understand -
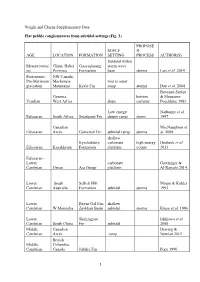
Wright and Cherns Supplementary Data Flat
Wright and Cherns Supplementary Data Flat pebble conglomerates from subtidal settings (Fig. 2) PROPOSE SHELF D AGE LOCATION FORMATION SETTING PROCESS AUTHOR(S) Subtidal within Mesoproteroz China, Hebei Gaoyuzhuang storm wave oic Province Formation base storms Luo et al. 2014 Proterozoic, NW Canada, Pre-Marinoan Mackenzie mid to outer glaciation Mountains Keele Fm ramp storms Day et al. 2004 Bertrand-Sarfati Gourma, bottom & Moussine- Vendian West Africa slope currents' Pouchkine 1983 Low energy Narbonne et al. Ediacaran South Africa Swartpunt Fm deeper ramp storm 1997 Canadian MacNaughton et Ediacaran Arctic Gametrail Fm subtidal ramp storms al. 2008 shallow Kyrshabakty carbonate high energy Heubeck et al. Ediacaran Kazakhstan Formation platform events 2013 Ediacaran - Lower carbonate Grotzinger & Cambrian Oman Ara Group platform Al-Rawahi 2014 Lower South Sellick Hill Mount & Kidder Cambrian Australia Formation subtidal storms 1993 Lower Bayan Gol Fm, shallow Cambrian W Mongolia Zavkhan Basin subtidal storms Kruse et al. 1996 Lower Shuijingtuo Ishikawa et al. Cambrian South China Fm subtidal 2008 Middle Canadian Dewing & Cambrian Arctic ramp Nowlan 2012 British Middle Columbia, Cambrian Canada Jubilee Fm Pope 1990 1 Middle Pratt & Cambrian Argentina La Laja Fm subtidal shelf tsunamis Bordonaro 2007 low energy Middle shallow Cambrian Australia Ranken Lst subtidal storms Kruse 1996 Middle Wyoming, Upper Gros Cambrian USA Ventre Shale Csonka 2009 middle upper Middle W Utah, upper Wheeler, carbonate belt - Cambrian USA Marjum fms subtidal shelf Robison 1964 Middle- Upper Supratidal to Cambrian NW China subtidal fpc storms Liang et al. 1993 Ust’- Brus,Labaz, Middle Orakta, Cambrian - Kulyumbe, Lower Ujgur and Iltyk Kouchinsky et Ordovician Siberia fms al. -

Appendix 3.Pdf
A Geoconservation perspective on the trace fossil record associated with the end – Ordovician mass extinction and glaciation in the Welsh Basin Item Type Thesis or dissertation Authors Nicholls, Keith H. Citation Nicholls, K. (2019). A Geoconservation perspective on the trace fossil record associated with the end – Ordovician mass extinction and glaciation in the Welsh Basin. (Doctoral dissertation). University of Chester, United Kingdom. Publisher University of Chester Rights Attribution-NonCommercial-NoDerivatives 4.0 International Download date 26/09/2021 02:37:15 Item License http://creativecommons.org/licenses/by-nc-nd/4.0/ Link to Item http://hdl.handle.net/10034/622234 International Chronostratigraphic Chart v2013/01 Erathem / Era System / Period Quaternary Neogene C e n o z o i c Paleogene Cretaceous M e s o z o i c Jurassic M e s o z o i c Jurassic Triassic Permian Carboniferous P a l Devonian e o z o i c P a l Devonian e o z o i c Silurian Ordovician s a n u a F y r Cambrian a n o i t u l o v E s ' i k s w o Ichnogeneric Diversity k p e 0 10 20 30 40 50 60 70 S 1 3 5 7 9 11 13 15 17 19 21 n 23 r e 25 d 27 o 29 M 31 33 35 37 39 T 41 43 i 45 47 m 49 e 51 53 55 57 59 61 63 65 67 69 71 73 75 77 79 81 83 85 87 89 91 93 Number of Ichnogenera (Treatise Part W) Ichnogeneric Diversity 0 10 20 30 40 50 60 70 1 3 5 7 9 11 13 15 17 19 21 n 23 r e 25 d 27 o 29 M 31 33 35 37 39 T 41 43 i 45 47 m 49 e 51 53 55 57 59 61 c i o 63 z 65 o e 67 a l 69 a 71 P 73 75 77 79 81 83 n 85 a i r 87 b 89 m 91 a 93 C Number of Ichnogenera (Treatise Part W) -

SVP's Letter to Editors of Journals and Publishers on Burmese Amber And
Society of Vertebrate Paleontology 7918 Jones Branch Drive, Suite 300 McLean, VA 22102 USA Phone: (301) 634-7024 Email: [email protected] Web: www.vertpaleo.org FEIN: 06-0906643 April 21, 2020 Subject: Fossils from conflict zones and reproducibility of fossil-based scientific data Dear Editors, We are writing you today to promote the awareness of a couple of troubling matters in our scientific discipline, paleontology, because we value your professional academic publication as an important ‘gatekeeper’ to set high ethical standards in our scientific field. We represent the Society of Vertebrate Paleontology (SVP: http://vertpaleo.org/), a non-profit international scientific organization with over 2,000 researchers, educators, students, and enthusiasts, to advance the science of vertebrate palaeontology and to support and encourage the discovery, preservation, and protection of vertebrate fossils, fossil sites, and their geological and paleontological contexts. The first troubling matter concerns situations surrounding fossils in and from conflict zones. One particularly alarming example is with the so-called ‘Burmese amber’ that contains exquisitely well-preserved fossils trapped in 100-million-year-old (Cretaceous) tree sap from Myanmar. They include insects and plants, as well as various vertebrates such as lizards, snakes, birds, and dinosaurs, which have provided a wealth of biological information about the ‘dinosaur-era’ terrestrial ecosystem. Yet, the scientific value of these specimens comes at a cost (https://www.nytimes.com/2020/03/11/science/amber-myanmar-paleontologists.html). Where Burmese amber is mined in hazardous conditions, smuggled out of the country, and sold as gemstones, the most disheartening issue is that the recent surge of exciting scientific discoveries, particularly involving vertebrate fossils, has in part fueled the commercial trading of amber. -

Dornbos.Web.CV
Stephen Quinn Dornbos Associate Professor and Department Chair Department of Geosciences University of Wisconsin-Milwaukee Milwaukee, WI 53201-0413 Phone: (414) 229-6630 Fax: (414) 229-5452 E-mail: [email protected] http://uwm.edu/geosciences/people/dornbos-stephen/ EDUCATION 2003 Ph.D., Geological Sciences, University of Southern California, Los Angeles, CA. 1999 M.S., Geological Sciences, University of Southern California, Los Angeles, CA. 1997 B.A., Geology, The College of Wooster, Wooster, OH. ADDITIONAL EDUCATION 2002 University of Washington, Summer Marine Invertebrate Zoology Course, Friday Harbor Laboratories. 1997 Louisiana State University, Summer Field Geology Course. PROFESSIONAL EXPERIENCE 2017-Present Department Chair, Department of Geosciences, University of Wisconsin-Milwaukee. 2010-Present Associate Professor, Department of Geosciences, University of Wisconsin-Milwaukee. 2004-2010 Assistant Professor, Department of Geosciences, University of Wisconsin-Milwaukee. 2012-Present Adjunct Curator, Geology Department, Milwaukee Public Museum. 2004-Present Curator, Greene Geological Museum, University of Wisconsin- Milwaukee. 2003-2004 Postdoctoral Research Fellow, Department of Earth Sciences, University of Southern California. 2002 Research Assistant, Invertebrate Paleontology Department, Natural History Museum of Los Angeles County. EDITORIAL POSITIONS 2017-Present Editorial Board, Heliyon. 2015-Present Board of Directors, Coquina Press. 2014-Present Commentaries Editor, Palaeontologia Electronica. 2006-Present Associate Editor, Palaeontologia Electronica. Curriculum Vitae – Stephen Q. Dornbos 2 RESEARCH INTERESTS 1) Evolution and preservation of early life on Earth. 2) Evolutionary paleoecology of early animals during the Cambrian radiation. 3) Geobiology of microbial structures in Precambrian–Cambrian sedimentary rocks. 4) Cambrian reef evolution, paleoecology, and extinction. 5) Exceptional fossil preservation. HONORS AND AWARDS 2013 UWM Authors Recognition Ceremony. 2011 Full Member, Sigma Xi. -

Permophiles Issue
Contents Notes from the SPS Secretary ...........................................................................................................................1 Shen Shuzhong Notes from the SPS Chair ..................................................................................................................................2 Charles M. Henderson Meeting Report: Report on the Continental Siena Meeting, Italy, September 2006.....................................3 G. Cassinis, A. Lazzarotto, P. Pittau Working Group Report: Short report on 2005-2006 activities of the non-marine – marine correlation work- ing group of SPS ..................................................................................................................................................5 J.W. Schneider Report of SPS Working Group on “Using Permian transitional biotas as gateways for global correlation”7 Guang R. Shi International Permian Time Scale ...................................................................................................................10 Voting Members of the SPS ............................................................................................................................. 11 Submission guideline for Issue 49 ....................................................................................................................12 Reports: Ostracods (Crustacea) from the Permian-Triassic boundary interval of South China (Huaying Mountains, eastern Sichuan Province): paleo-oxygenation significance .......................................................12 -

Diversity Variation, Distribution Pattern, and Evolutionary Implicat
+Model PALWOR-305; No. of Pages 20 ARTICLE IN PRESS Available online at www.sciencedirect.com ScienceDirect Palaeoworld xxx (2015) xxx–xxx A systematic overview of fossil osmundalean ferns in China: Diversity variation, distribution pattern, and evolutionary implications a,f,g b,c,∗ d b e Ning Tian , Yong-Dong Wang , Man Dong , Li-Qin Li , Zi-Kun Jiang a College of Palaeontology, Shenyang Normal University, Shenyang 110034, China b Nanjing Institute of Geology and Palaeontology, Chinese Academy of Sciences, Nanjing 210008, China c Key Laboratory of Economic Stratigraphy and Palaeogeography, Chinese Academy of Sciences, Nanjing 210008, China d College of Geosciences, Yangtze University, Wuhan 430100, China e Chinese Academy of Geological Sciences, Beijing 100037, China f State Key Laboratory of Palaeobiology and Stratigraphy, Nanjing Institute of Geology and Palaeontology, Chinese Academy of Sciences, Nanjing 210008, China g Key Laboratory for Evolution of Past Life in Northeast Asia, Ministry of Land and Resources, Shenyang 110034, China Received 7 August 2014; received in revised form 9 December 2014; accepted 12 May 2015 Abstract The order Osmundales is a unique fern taxon with extensive fossil records in geological past. Diverse osmundalean fossils have been reported from China, ranging in age from the Late Palaeozoic to the Cenozoic. Most of them are based on leaf impressions/compressions, but permineralized rhizomes are also well documented. In this study, we provide a systematic overview on fossil osmundalean ferns in China with special references on diversity variations, distribution patterns, and evolutionary implications. Fossil evidence indicates that this fern lineage first appeared in the Late Palaeozoic in China. -

Active Research Grants
Linda C. Ivany Professor Department of Earth and Environmental Sciences Heroy Geology Laboratory,Syracuse University, Syracuse, NY 13244 phone: (315) 443-3626 / fax: (315) 443-3363 / email: [email protected] http://thecollege.syr.edu/people/faculty/pages/ear/Ivany-Linda.html https://orcid.org/0000-0002-4692-3455 Education Ph.D. in Earth and Planetary Sciences, 1997, Harvard University Advisor: Stephen Jay Gould M.S. in Geology, minor in Zoology, 1990, University of Florida-Gainesville Advisor: Douglas S. Jones B.S. in Geology, minor in Zoology, 1988, Syracuse University Advisor: Cathryn R. Newton Academic Positions 2012-present Professor of Earth Sciences, Syracuse University 2005-2012 Associate Professor of Earth Sciences, Syracuse University 2001-2005 Assistant Professor of Earth Sciences, Syracuse University 2000-2001 Visiting Assistant Professor of Earth Sciences, Syracuse University 1997-2000 Michigan Society Fellow and Visiting Assistant Professor of Geological Sciences, University of Michigan General Research Interests Evolutionary Paleoecology, Paleoclimatology, Stable Isotopes in Paleobiology I am a marine paleoecologist and paleoclimatologist. My interests lie broadly in the evolution of the Earth-life system and how ecosystems and their component taxa evolve and respond to changes in the physical environment. Specific areas of interest include biotic and climatic change during the Paleogene (~65-24 million years ago); use of geochemical data, particularly stable isotopes, derived from accretionary biogenic materials for inference -

Lee-Riding-2018.Pdf
Earth-Science Reviews 181 (2018) 98–121 Contents lists available at ScienceDirect Earth-Science Reviews journal homepage: www.elsevier.com/locate/earscirev Marine oxygenation, lithistid sponges, and the early history of Paleozoic T skeletal reefs ⁎ Jeong-Hyun Leea, , Robert Ridingb a Department of Geology and Earth Environmental Sciences, Chungnam National University, Daejeon 34134, Republic of Korea b Department of Earth and Planetary Sciences, University of Tennessee, Knoxville, TN 37996, USA ARTICLE INFO ABSTRACT Keywords: Microbial carbonates were major components of early Paleozoic reefs until coral-stromatoporoid-bryozoan reefs Cambrian appeared in the mid-Ordovician. Microbial reefs were augmented by archaeocyath sponges for ~15 Myr in the Reef gap early Cambrian, by lithistid sponges for the remaining ~25 Myr of the Cambrian, and then by lithistid, calathiid Dysoxia and pulchrilaminid sponges for the first ~25 Myr of the Ordovician. The factors responsible for mid–late Hypoxia Cambrian microbial-lithistid sponge reef dominance remain unclear. Although oxygen increase appears to have Lithistid sponge-microbial reef significantly contributed to the early Cambrian ‘Explosion’ of marine animal life, it was followed by a prolonged period dominated by ‘greenhouse’ conditions, as sea-level rose and CO2 increased. The mid–late Cambrian was unusually warm, and these elevated temperatures can be expected to have lowered oxygen solubility, and to have promoted widespread thermal stratification resulting in marine dysoxia and hypoxia. Greenhouse condi- tions would also have stimulated carbonate platform development, locally further limiting shallow-water cir- culation. Low marine oxygenation has been linked to episodic extinctions of phytoplankton, trilobites and other metazoans during the mid–late Cambrian. -

The Ediacaran–Cambrian Transition: the Emerging Record from Small Carbonaceous Fossils (Scfs)
38 The Ediacaran–Cambrian transition: the emerging record from Small Carbonaceous Fossils (SCFs) Ben J. Slater1, Thomas H.P. Harvey2, Romain Guilbaud3, Sebastian Willman1, Graham E. Budd1, Nicholas J. Butterfield4. 1Department of Earth Sciences (Palaeobiology), Uppsala University, Villavägen 16, SE-75236 Uppsala, Sweden ([email protected]) 2School of Geography, Geology and the Environment, University of Leicester, Leicester LE1 7RH, UK 3Lancaster Environment Centre, Lancaster University, Lancaster, LA1 4YQ, UK. 4Department of Earth Sciences, University of Cambridge, Downing Street, Cambridge, CB2 3EQ, UK Abstract.––The most profound change in the fossil record is centered on the Ediacaran–Cambrian transition. Sediments deposited near to this boundary record the first complex trace fossils, the first animal biomineralizers and the earliest fossils of the major animal groups that came to define the subsequent Phanerozoic fossil record. Here we discuss how the emerging record of small carbonaceous fossils (SCFs) is shedding new light on unmineralized aspects of the biota from this critical evolutionary transition. The new SCFs record complements currently established records from trace fossils and biomineralized shelly-remains in reconstructing the broader scale macroevolutionary patterns of this transition, but via an entirely different taphonomic window. SCFs extend the record of multiple major animal groups, improve our picture of protistan diversity from this interval, and reveal new data on elements of the biota that span the Ediacaran–Cambrian boundary. Key words: small carbonaceous fossils; bilateria; Burgess Shale-type preservation; Ediacaran; Terreneuvian. The dramatic changes in the fossil record across subsequent Cambrian radiation (Butterfield, the Ediacaran–Cambrian transition seemingly 2003; Slater et al., 2018a). -

Ediacaran and Cambrian Stratigraphy in Estonia: an Updated Review
Estonian Journal of Earth Sciences, 2017, 66, 3, 152–160 https://doi.org/10.3176/earth.2017.12 Ediacaran and Cambrian stratigraphy in Estonia: an updated review Tõnu Meidla Department of Geology, Institute of Ecology and Earth Sciences, Faculty of Science and Technology, University of Tartu, Ravila 14a, 50411 Tartu, Estonia; [email protected] Received 18 December 2015, accepted 18 May 2017, available online 6 July 2017 Abstract. Previous late Precambrian and Cambrian correlation charts of Estonia, summarizing the regional stratigraphic nomenclature of the 20th century, date back to 1997. The main aim of this review is updating these charts based on recent advances in the global Precambrian and Cambrian stratigraphy and new data from regions adjacent to Estonia. The term ‘Ediacaran’ is introduced for the latest Precambrian succession in Estonia to replace the formerly used ‘Vendian’. Correlation with the dated sections in adjacent areas suggests that only the latest 7–10 Ma of the Ediacaran is represented in the Estonian succession. The gap between the Ediacaran and Cambrian may be rather substantial. The global fourfold subdivision of the Cambrian System is introduced for Estonia. The lower boundary of Series 2 is drawn at the base of the Sõru Formation and the base of Series 3 slightly above the former lower boundary of the ‘Middle Cambrian’ in the Baltic region, marked by a gap in the Estonian succession. The base of the Furongian is located near the base of the Petseri Formation. Key words: Ediacaran, Cambrian, correlation chart, biozonation, regional stratigraphy, Estonia, East European Craton. INTRODUCTION The latest stratigraphic chart of the Cambrian System in Estonia (Mens & Pirrus 1997b, p. -

GSSP) of the Drumian Stage (Cambrian) in the Drum Mountains, Utah, USA
Articles 8585 by Loren E. Babcock1, Richard A. Robison2, Margaret N. Rees3, Shanchi Peng4, and Matthew R. Saltzman1 The Global boundary Stratotype Section and Point (GSSP) of the Drumian Stage (Cambrian) in the Drum Mountains, Utah, USA 1 School of Earth Sciences, The Ohio State University, 125 South Oval Mall, Columbus, OH 43210, USA. Email: [email protected] and [email protected] 2 Department of Geology, University of Kansas, Lawrence, KS 66045, USA. Email: [email protected] 3 Department of Geoscience, University of Nevada, Las Vegas, Las Vegas, NV 89145, USA. Email: [email protected] 4 State Key Laboratory of Palaeobiology and Stratigraphy, Nanjing Institute of Geology and Palaeontology, Chinese Academy of Sciences, 39 East Beijing Road, Nanjing 210008, China. Email: [email protected] The Global boundary Stratotype Section and Point correlated with precision through all major Cambrian regions. (GSSP) for the base of the Drumian Stage (Cambrian Among the methods that should be considered in the selection of a GSSP (Remane et al., 1996), biostratigraphic, chemostratigraphic, Series 3) is defined at the base of a limestone (cal- paleogeographic, facies-relationship, and sequence-stratigraphic cisiltite) layer 62 m above the base of the Wheeler For- information is available (e.g., Randolph, 1973; White, 1973; McGee, mation in the Stratotype Ridge section, Drum Moun- 1978; Dommer, 1980; Grannis, 1982; Robison, 1982, 1999; Rowell et al. 1982; Rees 1986; Langenburg et al., 2002a, 2002b; Babcock et tains, Utah, USA. The GSSP level contains the lowest al., 2004; Zhu et al., 2006); that information is summarized here. occurrence of the cosmopolitan agnostoid trilobite Pty- Voting members of the International Subcommission on Cam- chagnostus atavus (base of the P.