Delaware River Biomonitoring Program Quality Assurance Project Plan
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

DETERMINATION of TRAVELTIME in the DELAWARE RIVER, HANCOCK, NEW YORK, to the DELAWARE WATER GAP by USE of a CONSERVATIVE DYE TRACER by Kirk E
DETERMINATION OF TRAVELTIME IN THE DELAWARE RIVER, HANCOCK, NEW YORK, TO THE DELAWARE WATER GAP BY USE OF A CONSERVATIVE DYE TRACER by Kirk E. White and Todd W. Kratzer__________________ U.S. GEOLOGICAL SURVEY Water-Resources Investigations Report 93-4203 Prepared in cooperation with the DELAWARE RIVER BASIN COMMISSION Lemoyne, Pennsylvania 1994 U.S. DEPARTMENT OF THE INTERIOR BRUCE BABBIT, Secretary U.S. GEOLOGICAL SURVEY Robert M. Hirsh, Acting Director For additional information Copies of this report may be write to: purchased from: U.S. Geological Survey Earth Science Information Center District Chief Open-File Reports Section U.S. Geological Survey Box 25286, MS 517 840 Market Street Denver Federal Center Lemoyne, Pennsylvania 17043-1586 Denver, Colorado 80225 CONTENTS Page Abstract....................................................................................1 Introduction ................................................................................1 Purpose and scope....................................................................1 Acknowledgments....................................................................4 Description of study reach .............................................................5 Methods of data collection and analysis .................................................5 Field and laboratory procedures.................................................5 Data analysis .................................................................8 River discharge.............................................................................16 -

Delaware River Basin Wild and Scenic River Values
DELAWARE RIVER BASIN NATIONAL PARK SERVICE | NATIONAL WILD AND SCENIC RIVERS SYSTEM | US DEPARTMENT OF THE INTERIOR NATIONAL WILD AND SCENIC RIVER VALUES DELAWARE RIVER BASIN | NATIONAL WILD AND SCENIC RIVER VALUES DELAWARE RIVER BASIN DELAWARE | NATIONAL WILD AND SCENIC RIVER VALUES NATIONAL Photo: David B. Soete PENNSYLVANIA • NEW YORK • NEW JERSEY EXPERIENCE YOUR AMERICA NATIONAL PARK SERVICE | NATIONAL WILD AND SCENIC RIVERS SYSTEM | US DEPARTMENT OF THE INTERIOR DELAWARE RIVER BASIN CONTENTS WILD AND SCENIC RIVER VALUES PENNSYLVANIA, NEW YORK, AND NEW JERSEY Overview ....................................... 2 Lower Delaware National Wild and Scenic River ....... 48 Dear friends of the Delaware River Basin, The Wild and Scenic Rivers Act . 5 ° Cultural . 50 Outstandingly Remarkable Values . 8 ~ Lower Delaware River (including Tinicum Creek, What comes to mind when you think about the Delaware River and its tributaries? Tohickon Creek, and Paunacussing Creek) . 50 ° Cultural Values . 10 Do you think of a beautiful river valley where one can experience solitude in nature and Ecological Values . 12 ° Ecological . 51 enjoy the incredible scenery of the meandering river and forested hillsides? A family friendly ° ~ Lower Delaware River . 51 destination that provides great opportunities for boating, fi shing, and picnicking? A rare example ° Geological Values . 14 ~ Tinicum Creek . 52 of a large, undammed river that allows continual natural migration of native fi sh from the sea to ° Recreational Values . 16 the river headwaters and back? An intact network of aquatic and riparian habitat that supports Scenic Values . 17 ~ Tohickon Creek . 52 abundance and diversity of plants, fi sh, birds, and other animals? A waterway and valley with an ° ~ Paunacussing Creek . -

Monitoring of Tributaries
SCENIC RIVERS MONITORING PROGRAM FOR THE NON-TIDAL DELAWARE RIVER: QUALITY ASSURANCE PROJECT PLAN 2008-2009 DELAWARE RIVER BASIN COMMISSION DRBC Project Officer: Thomas J. Fikslin Date DRBC Quality Assurance Officer: Edward D. Santoro Date DRBC Program Officer Robert L. Limbeck Date USEPA Project Officer Patricia Iraci Date Table of Contents 1 TABLE OF CONTENTS .................................................................................................................................... 1 2 PROJECT MANAGEMENT ELEMENTS .................................................................................................. 1-1 2.1 DISTRIBUTION LIST .................................................................................................................................... 1-1 2.2 PROJECT / TASK ORGANIZATION ................................................................................................................ 1-2 2.3 PROBLEM DEFINITION AND BACKGROUND ................................................................................................. 1-4 2.4 PROJECT / TASK DESCRIPTION .................................................................................................................... 1-7 2.5 QUALITY OBJECTIVES AND CRITERIA ......................................................................................................... 1-7 2.5.1 Precision ............................................................................................................................................ 1-7 2.5.2 Accuracy ........................................................................................................................................... -

Delaware River Basin
DELAWARE RIVER BASIN NATIONAL PARK SERVICE | NATIONAL WILD AND SCENIC RIVERS SYSTEM | US DEPARTMENT OF THE INTERIOR NATIONAL WILD AND SCENIC RIVER VALUES DELAWARE RIVER BASIN | NATIONAL WILD AND SCENIC RIVER VALUES DELAWARE RIVER BASIN DELAWARE | NATIONAL WILD AND SCENIC RIVER VALUES NATIONAL Photo: David B. Soete PENNSYLVANIA • NEW YORK • NEW JERSEY EXPERIENCE YOUR AMERICA NATIONAL PARK SERVICE | NATIONAL WILD AND SCENIC RIVERS SYSTEM | US DEPARTMENT OF THE INTERIOR DELAWARE RIVER BASIN CONTENTS WILD AND SCENIC RIVER VALUES PENNSYLVANIA, NEW YORK, AND NEW JERSEY Overview ....................................... 4 Lower Delaware National Wild and Scenic River ....... 50 Dear friends of the Delaware River Basin, The Wild and Scenic Rivers Act . 7 ° Cultural . 52 Outstandingly Remarkable Values . 10 ~ Lower Delaware River (including Tinicum Creek, What comes to mind when you think about the Delaware River and its tributaries? Tohickon Creek, and Paunacussing Creek) . 52 ° Cultural Values . 12 Do you think of a beautiful river valley where one can experience solitude in nature and Ecological Values . 14 ° Ecological . 53 enjoy the incredible scenery of the meandering river and forested hillsides? A family friendly ° ~ Lower Delaware River . 53 destination that provides great opportunities for boating, fi shing, and picnicking? A rare example ° Geological Values . 16 ~ Tinicum Creek . 54 of a large, undammed river that allows continual natural migration of native fi sh from the sea to ° Recreational Values . 18 the river headwaters and back? An intact network of aquatic and riparian habitat that supports Scenic Values . 19 ~ Tohickon Creek . 54 abundance and diversity of plants, fi sh, birds, and other animals? A waterway and valley with an ° ~ Paunacussing Creek . -

Drbc Delaware River Biomonitoring Program
DRBC DELAWARE RIVER BIOMONITORING PROGRAM 2006-2007 QUALITY ASSURANCE PROJECT PLAN FINAL DRAFT: MARCH 7, 2007. DELAWARE RIVER BASIN COMMISSION DRBC Project Officer: Thomas J. Fikslin Date DRBC Quality Assurance Officer: Edward D. Santoro Date DRBC Program Officer Robert L. Limbeck Date USEPA Project Officer Date i TABLE OF CONTENTS 1. Project Management .................................................................................................................................. 1 1.1 Distribution List................................................................................................................................. 1 1.2 Project/ Task Organization................................................................................................................. 1 1.3 Problem Definition/ Background........................................................................................................ 4 1.4 Project Task/ Description ................................................................................................................... 5 1.5 Quality Objectives and Criteria for Measurement Data....................................................................... 6 1.5.1 Bias............................................................................................................................................ 6 1.5.2 Precision .................................................................................................................................... 6 1.5.3 Completeness ............................................................................................................................ -

West Branch Delaware River and Tributaries
West Branch Delaware River and Tributaries Delaware River Basin Commission Page 26 3448 BCP Oquaga Creek, NY Delaware River Basin Commission Page 27 3448 BCP Oquaga Creek at Mill St., Deposit, NY Broome County, NY. USGS Site No. 01426000. Latitude 42.059381 Longitude -75.426851 by Map Interpolation NAD83 decimal degrees. Population of Watershed: 2000: 1,346 2010: 1,303 Drainage Area: 67.94 square miles, tributary to West Branch Delaware River Zone W (West Branch) EWQ definition currently underway by USGS/NPS, anticipated Completion by 2018 This tributary to the West Branch Delaware River was added to the list of control points because of necessity to establish baseline water quality conditions prior to potential natural gas development activities. Nearest upstream Interstate Control Point: None; compare with West Branch Delaware River at Stilesville, which is sited just below Cannonsville Reservoir (NYSDEC has data for Cannonsville release water quality) Nearest downstream receiving Interstate Control Point: West Branch Delaware River at Hale Eddy. Known dischargers within watershed: Undefined Watershed is 85.5 % forested; urban land cover is 0.8%. 100% glaciated. No carbonate rock. Mean annual precipitation 44.5 inches. (http://water.usgs.gov/osw/streamstats/, accessed 2012). Flow Statistics: Max Flow 90% Flow 75% 60% 50% 40% 25% Flow 10% Flow (CFS) Min (CFS) (CFS) Flow Flow Flow Flow (CFS) Flow (CFS) (CFS) (CFS) (CFS) (CFS) 6,659.47 304.55 165.92 107.14 83.37 51.45 29.06 13.36 2.27 StreamStats Low-Flow Stream Statistics M7D2Y (ft³/s) -

Town of Delaware, NY Callicoon Water District Map, Plan and Report
Town of Delaware, NY Callicoon Water District Map, Plan and Report TEXT 1.0 INTRODUCTION…………………………………………………………………………………………….………1 2.0 EXISTING COMMUNITY WATER SYSTEM……………………………………………………….……… 3 3.0 PROPOSED NEW DISTRICT…………………………………………………………………………….……….7 4.0 DESCRIPTION OF FACILITIES – NEEDED IMPROVEMENTS…………………………….………….7 5.0 PROPOSED DISTRICT OPERATIONS………………………………………………………………….…...10 6.0 REGULATORY REVIEW AND APPROVAL REQUIRED PRIOR TO CONSTRUCTION….....11 7.0 MAXIMUM AMOUNT TO BE EXPENDED…………………………………………………………….….11 8.0 DETAILED EXPLANANTION OF COSTS……………………………………………………………….…...12 9.0 COST TO THE TYPICAL PROPERTY………………………………………………………………………....15 10.0 METHOD OF FINANCE……………………………………………………………………………………….....16 11.0 STATEMENT AS TO BENEFIT ASSESSMENT………………………………………………..….…..…..16 APPENDICES April 2, 2018 i Town of Delaware, NY Callicoon Water District Map, Plan and Report Appendices Appendix A Callicoon Water Company – NYS Department of State Incorporation Record Appendix B NYS EFC DWSRF 2018 IUP Listing Appendix C Water System Map Proposed Water District Map Proposed Water District Parcels Appendix D Complete SEQR Packet with Resolutions Appendix E NYS Comptroller’s Office 2018 Special Districts Memo • Notice of Hearing Requirements for the establishments of town Special Districts • FAQs on the Establishment of Town Special Districts Appendix F Sullivan County Office of Real Property Tax Services Parcel Reports Appendix G FEMA Map Panel No. 36105C0243F Appendix H Formation, Financing, and Implementation Schedule Appendix I Hardship Letter -

Delaware River Basin
DELAWARE RIVER BASIN NATIONAL PARK SERVICE | NATIONAL WILD AND SCENIC RIVERS SYSTEM | US DEPARTMENT OF THE INTERIOR NATIONAL WILD AND SCENIC RIVER VALUES DELAWARE RIVER BASIN | NATIONAL WILD AND SCENIC RIVER VALUES DELAWARE RIVER BASIN DELAWARE | NATIONAL WILD AND SCENIC RIVER VALUES NATIONAL Photo: David B. Soete PENNSYLVANIA • NEW YORK • NEW JERSEY EXPERIENCE YOUR AMERICA NATIONAL PARK SERVICE | NATIONAL WILD AND SCENIC RIVERS SYSTEM | US DEPARTMENT OF THE INTERIOR DELAWARE RIVER BASIN CONTENTS WILD AND SCENIC RIVER VALUES PENNSYLVANIA, NEW YORK, AND NEW JERSEY Overview ....................................... 2 Lower Delaware National Wild and Scenic River ....... 48 Dear friends of the Delaware River Basin, The Wild and Scenic Rivers Act . 5 ° Cultural . 50 Outstandingly Remarkable Values . 8 ~ Lower Delaware River (including Tinicum Creek, What comes to mind when you think about the Delaware River and its tributaries? Tohickon Creek, and Paunacussing Creek) . 50 ° Cultural Values . 10 Do you think of a beautiful river valley where one can experience solitude in nature and Ecological Values . 12 ° Ecological . 51 enjoy the incredible scenery of the meandering river and forested hillsides? A family friendly ° ~ Lower Delaware River . 51 destination that provides great opportunities for boating, fi shing, and picnicking? A rare example ° Geological Values . 14 ~ Tinicum Creek . 52 of a large, undammed river that allows continual natural migration of native fi sh from the sea to ° Recreational Values . 16 the river headwaters and back? An intact network of aquatic and riparian habitat that supports Scenic Values . 17 ~ Tohickon Creek . 52 abundance and diversity of plants, fi sh, birds, and other animals? A waterway and valley with an ° ~ Paunacussing Creek . -
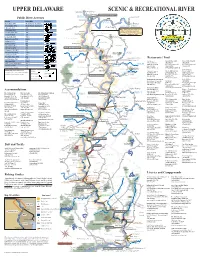
Map Inside.Pmd
UPPER DELAWARE L% SCENIC & RECREATIONAL RIVER Balls Eddy To Binghamton )"17 Hancock West Branch Hancock/Buckingham Bridge Public River Accesses Delaware River " To Downsville 17 )"30 ShehawkenL% )" ¨¦§86 HHV 10V` 1CV :H1C1 1V !( East Branch L% Delaware River :CCRR7 ^ _ V `:JH.VC:1:`V 330 Hancock (! East Branch .V.:1@VJ^ _ V `:JH.VC:1:`V (! )"17 To Roscoe )"370 Beaverkill River :JHQH@ ^_ Fishs Eddy Note: Most land within the river QH@]Q` ^ _ % ! StockportL corridor is privately owned and %H@1J$.:I^ _ 8 )"97 should be treated with due respect. Stockport Town of (Q`R01CCV^_ (! Please don't trespass or litter. 191 French Woods (QJ$RR7^_ 8 )" State Forest Preserve :@V +`VV@^_ 8 Buckingham 325 "28 ,VCC:I^_ !( ) Abe Lord Creek +:CC1HQQJ^ _ BuckinghamL% +QH.VH QJ^_ )"97 Hancock Lordville/Equinunk Bridge LordvilleL% :I:H%^ _ (!" Lordville 320 Bouchoux Trail @1JJV`/:CC^_ 8 !( Township Equinunk (! :``Q1G%`$^_ 8 ! Basket Creek Rock Valley State North Branch :`G7 Q1J^ _ 8 Forest Preserve Equinunk Creek Bouchoux Trail 1VJ21CV310V`^_ State Forest Preserve (:H@:1:6VJ^ _ 8 Long Eddy Pool Delaware County, NY Restaurants / Food ! Sullivan County, NY 1$.C:JR^_ 8 Calder House !( Long Eddy Cafe Devine * Gerard’s River Grill Nora’s Luvin’ Spoonful 315 (! Museum L% 33 Lower Main St. 251 Bridge St. 141 Kirk Rd. +Q`11J/:`I^_ 8 Long Eddy 134 Basket Creek )"191 Basket Historical UV East Branch Callicoon, NY 12723 Narrowsburg, NY 12764 Narrowsburg, NY 12764 Society 2QJ$:%]^_ L% (845) 887-3076 (845) 252-6562 (845) 252-3891 1018 Basket Creek ]:``Q1G%.^_ Manchester Township UV Crystal Lake www.cafedevine.com www.gerardsrivergrill.com Town of State Reservation Peck’s Market * CV:VQ V7QIV`:H1C1 1V :JR (! L% :`@1J$ `: . -

Upper Delaware Scenic and Recreational River New York, Pennsylvania February 2014
NATIONAL PARK SERVICE • U.S. DEPARTMENT OF THE INTERIOR Foundation Document Upper Delaware Scenic and Recreational River New York, Pennsylvania February 2014 Photo by David B. Soete Foundation Document From Hancock, NY to Binghamton, NY From Hancock, NY 43 miles 0 25 Kilometers to Albany, NY 134 miles D N.Y. e 0 25 Miles la Pa. w Kingston a Hancock r 17 North e Upper Delaware 97 52 209 Scenic and Monticello 87 81 Recreational N.Y. River 17B Thruway Honesdale (toll) River 652 Narrowsburg Carbondale Pa. Wurtsboro Lackawaxen 6 Hawley Barryville Newburgh 6 River Middletown 17 84 Port Jervis Scranton Milford Delaware Matamoras Hudson NE Extension Water Gap Pa. Turnpike National N.Y. (toll) Recreation 209 206 N.J. 380 Area 23 15 From Port Jervis, NY Stroudsburg to New York City, NY 476 75 miles From Port Jervis, NY 80 to Philadelphia, PA 33 140 miles Upper Delaware Scenic and Recreational River Contents Mission of the National Park Service 1 Introduction 2 Part 1: Core Components 3 Brief Description of the Upper Delaware Scenic and Recreational River 3 Purpose Statement 6 Significance Statements 7 Fundamental Resources and Values 8 Interpretive Themes 10 Part 2: Dynamic Components 11 Special Mandates and Administrative Commitments 11 Assessment of Planning and Data Needs 13 Analysis of Fundamental Resources and Values 13 Identification of Key Issues 13 Planning and Data Needs 14 Part 3: Contributors 21 Appendixes 22 Appendix A: Fundamental Resource and Values Analysis Tables for Upper Delaware Scenic and Recreational River 22 Appendix B: Enabling -

Foundation Document, Upper Delaware Scenic and Recreational
NatioNal Park Service • U.S. DePartmeNt of the iNterior Foundation Document Upper Delaware Scenic and Recreational River New York, Pennsylvania February 2014 Photo by David B. Soete Foundation Document From Hancock, NY to Binghamton, NY From Hancock, NY 43 miles 0 25 Kilometers to Albany, NY 134 miles D N.Y. e 0 25 Miles la Pa. w Kingston a Hancock r 17 North e Upper Delaware 97 52 209 Scenic and Monticello 87 81 Recreational N.Y. River 17B Thruway Honesdale (toll) River 652 Narrowsburg Carbondale Pa. Wurtsboro Lackawaxen 6 Hawley Barryville Newburgh 6 River Middletown 17 84 Port Jervis Scranton Milford Delaware Matamoras Hudson NE Extension Water Gap Pa. Turnpike National N.Y. (toll) Recreation 209 206 N.J. 380 Area 23 15 From Port Jervis, NY Stroudsburg to New York City, NY 476 75 miles From Port Jervis, NY 80 to Philadelphia, PA 33 140 miles Upper Delaware Scenic and Recreational River Contents Mission of the National Park Service 1 Introduction 2 Part 1: Core Components 3 Brief Description of the Upper Delaware Scenic and Recreational River 3 Purpose Statement 6 Significance Statements 7 Fundamental Resources and Values 8 Interpretive Themes 10 Part 2: Dynamic Components 11 Special Mandates and Administrative Commitments 11 Assessment of Planning and Data Needs 13 Analysis of Fundamental Resources and Values 13 Identification of Key Issues 13 Planning and Data Needs 14 Part 3: Contributors 21 Appendixes 22 Appendix A: Fundamental Resource and Values Analysis Tables for Upper Delaware Scenic and Recreational River 22 Appendix B: Enabling -

Existing Water Quality Atlas of the Delaware River Special Protection Waters
Existing Water Quality Atlas of the Delaware River Special Protection Waters DRBC Special Protection Waters Program September 2016 – Edition 1.0 Table of Contents Acknowledgments ..................................................................................................................... 6 Abbreviations ............................................................................................................................. 7 Introduction ............................................................................................................................... 8 Methods .................................................................................................................................. 14 Data Sufficiency for Existing Water Quality Definition ............................................................. 16 DRBC Usage of Existing Water Quality Tables .......................................................................... 17 Reach-Wide vs. Site-Specific Existing Water Quality ................................................................ 18 Monitoring and Data Quality Improvements since 1992.............................................................................................. 18 What Comes Next .................................................................................................................... 23 References ............................................................................................................................... 24 West Branch Delaware River and Tributaries ..........................................................................