Evaluation of Ground Water Quality of MIDC Area, Roha Through Water
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Seminar Report
Two day Awareness Seminar on Green Chemistry & Engineering Date : 22ⁿ - 23 April, 2014 Venue : Dr. Babasaheb Ambedkar University (DBATU), Lonere, Raigad Seminar Report Supporters : Department of Chemical and Petrochemicals, Government Of India INTRODUCTION The Two-day Awareness Seminar on Green Chemistry & Engineering, organised on the occasion of World Earth Day (22nd April), at Dr. Babasaheb Ambedkar Technological University (DBATU), Lonere, Dist. Raigad, was successfully concluded on 23rd April 2014. The objecves of the Seminar were as follows: - To create awareness of Green Chemistry and Engineering, with specific focus on the 12 Principles of Green Chemistry - To introduce emerging trends and technologies on green chemistry and engineering - To connect the parcipants to various soluon and technology provider companies - To learn from success case-studies and industry sharing ORGANISERS, SUPPORTERS & PARTNERS The Seminar was jointly organised by the Indian Chemical Council (ICC) and the Green ChemisTree Foundaon (GCF) with support from Dept. of Chemicals & Petrochemicals (DCPC), Govt. of India; Maharashtra Polluon Control Board (MPCB); Mahad Manufacturers Associaon (MMA); Roha Industries Associaon (RIA); and Industry Partners such as Clariant Chemicals India Pvt. Ltd and Pidilite Industries Ltd. PARTICIPATION The Seminar was well aended by over 80 Plant, Operaons and EHS personnel of more than 50 chemical companies located in the largest Industrial corridor of Maharashtra. The representaon was fairly distributed amongst Chiplun, Lote, Mahad, Roha, Patalganga and Taloja Industrial associaons, largely consisng of companies from Mahad and Roha regions. The companies parcipated were a mix of MNC (20%), Large (30%) and SME categories (50%). SPEAKERS, CONTEXT & CONTENT Leading academia experts who contributed to the deliberaon includes Prof. -
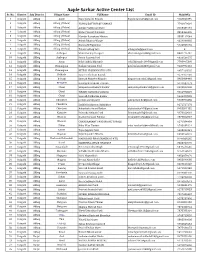
Aaple Sarkar Active Center List Sr
Aaple Sarkar Active Center List Sr. No. District Sub District Village Name VLEName Email ID MobileNo 1 Raigarh Alibag Akshi Sagar Jaywant Kawale [email protected] 9168823459 2 Raigarh Alibag Alibag (Urban) VISHAL DATTATREY GHARAT 7741079016 3 Raigarh Alibag Alibag (Urban) Ashish Prabhakar Mane 8108389191 4 Raigarh Alibag Alibag (Urban) Kishor Vasant Nalavade 8390444409 5 Raigarh Alibag Alibag (Urban) Mandar Ramakant Mhatre 8888117044 6 Raigarh Alibag Alibag (Urban) Ashok Dharma Warge 9226366635 7 Raigarh Alibag Alibag (Urban) Karuna M Nigavekar 9922808182 8 Raigarh Alibag Alibag (Urban) Tahasil Alibag Setu [email protected] 0 9 Raigarh Alibag Ambepur Shama Sanjay Dongare [email protected] 8087776107 10 Raigarh Alibag Ambepur Pranit Ramesh Patil 9823531575 11 Raigarh Alibag Awas Rohit Ashok Bhivande [email protected] 7798997398 12 Raigarh Alibag Bamangaon Rashmi Gajanan Patil [email protected] 9146992181 13 Raigarh Alibag Bamangaon NITESH VISHWANATH PATIL 9657260535 14 Raigarh Alibag Belkade Sanjeev Shrikant Kantak 9579327202 15 Raigarh Alibag Beloshi Santosh Namdev Nirgude [email protected] 8983604448 16 Raigarh Alibag BELOSHI KAILAS BALARAM ZAVARE 9272637673 17 Raigarh Alibag Chaul Sampada Sudhakar Pilankar [email protected] 9921552368 18 Raigarh Alibag Chaul VINANTI ANKUSH GHARAT 9011993519 19 Raigarh Alibag Chaul Santosh Nathuram Kaskar 9226375555 20 Raigarh Alibag Chendhre pritam umesh patil [email protected] 9665896465 21 Raigarh Alibag Chendhre Sudhir Krishnarao Babhulkar -

1 DR. BABASAHEB AMBEDKAR TECHNOLOGICAL UNIVERSITY LONERE, RAIGAD DISTRICT, MAHARASHTRA DBATU/Esst/COVID-19/2019-2020 March 1
DR. BABASAHEB AMBEDKAR TECHNOLOGICAL UNIVERSITY LONERE, RAIGAD DISTRICT, MAHARASHTRA DBATU/Esst/COVID-19/2019-2020 March 15, 2020 IMPORTANT OFFICE NOTIFICATION Ref: 1. Letter from Principal Secretary, Govt. of Maharashtra, No.Carona-2020/L.N.58/Arogya-5, dated 14-03-2020. 2. Letter from Higher and Technical Education Dept. No. Sankirna-2020(S/Kra.(114/20)/ vishi-1, dated 14-03-2020. 3. Letter from DTE , No.2-A/ADM/2020/148 dated 14-03-2020. The undersigned is to convey that because of the prevailing conditions arising out of the COVID-19 pandemic in the country and the subsequent advisory issued by the Government of Maharashtra (Vide References mentioned above) the University Administration has decided that: 1. All Academic, Co-curricualar, Extra-curricular, Games & Sports, activities of the University and All Affiliated Institutions shall remain suspended with immediate effect till 31-03-2020. 2. The Hostels shall remain closed with immediate effect and all (UG, PG, PhD and Diploma) students of the University Campus and Affiliated Institutions shall vacate the hostels and proceed to their homes on or before 18th March 2020 without fail and wait for further instructions to return to the hostels. 3. The Internal/Mid-Semester examinations of the University are suspended till March 31, 2020. 4. All functions/programs and social/cultural gatherings, Seminars / Conferences / Workshops / FDPs / Training Programs, etc. in the campus as well as Affiliated Institutions shall remain suspended till March 31, 2020. 5. Sudents are advised to visit the University Website (www.dbatu.ac.in) regularly for revised academic calendar and other updates. -

Yogesh-Patil-Resume 1.Pdf
YOGESH N. PATIL Director, Board of Examination & Evaluation, Dr. Babasaheb Ambedkar Marathwada University, Aurangabad (MH), India. Mobile : 8888092984 8830686800 Fax: 91-2140- 275040 (Office) E-mail: [email protected] Working as Director, Board of Examinations & Evaluation in Dr. Babasaheb Ambedkar Marathwada University, Aurangabad. Have worked as Controller of Examinations of Dr. B. A. Technological University, Lonere–Raigad Maharashtra, for more than Five Years and Associate Controller of Examinations for more than five years.. Successfully implemented the ICT based reforms suggested by Agrawal Committee for Examinations. Dr.B.A.Technological University is the first University in the State of Maharashtra to implement Online Thesis Processing for PhD. Represented Dr.B.A. Technological University in World Education Summit held on Feb 7 and Feb 8 2016 at The Oberoi, Dubai and won the prize under the category Collating Best Practices under Higher Education. Represented Dr.B.A.Technological University in Leaders Speak Education Summit at New Delhi in December 2015 and won the prize for Best in House ERP practices. Successfully conducted Winter 2017 examinations across Maharashtra for Engineering, Pharmacy and Architecture across 70 affiliated colleges. Experience Summary (total 24+ years) Five Years+ of Experience as Controller of Examinations at Dr.B.A.Technological University, Lonere Five+ yearsof experience as Associate Controller of Examination at Dr.B.A.TechnologicalUniversity, Lonere. Working as Additional Controller of Examinations at Dr.B.A.Technological University, Lonere. Nine month experience as Computer Programmer. Twenty Five years of total experience years (Lecturer/Sr.Lecturer/Sel Grade in pay band 4) in Computer Engineering. Two year of experience of In-charge Head Dept. -

Self Study Report
MANGAON TALUKA EDUCATION SOCIETYS’S DOSHI VAKIL ARTS & Goregaon Co – Operative Urban Bank Science & Commerce College GOREGAON – RAIGAD. (402 103) Est. 1998 (Affiliated to Mumbai University) Ph. (02140) 250348 Shri. Ramanlal Narayan Sheth Dr. G.D.Giri Shri. Dilip Nathuram Sheth President Principal Chairman To, The Director, National Assessment and Accreditation Council (NAAC), P.O. Box. 1075, Nagarbhavi, Bangalore – 560 072, Karnataka (India) Subject: Submission of Self Study Report. Dear Sir, We take this opportunity to submit our Self Study Report to the National Assessment and Accreditation Council (NAAC) for assessment and accreditation. This Self Study Report is prepared by us with honesty and sincere efforts as per the directive guidelines formulated by NAAC. In this report, we have highlighted our strengths and achievements in which we have succeeded within the last five years of our college. It is a privilege and matter of great pride for us to get accredited by an esteemed body like NAAC. NAAC as an autonomous body has proved as a noble assessment institution in maintaining quality excellence in the field of higher education of India for the last 20 years. Hope that our Self Study Report will satisfy your requirements and expectations. We will be grateful to you if you convey your useful suggestions. Thank you. Sincerely yours Sd/- Dr. G. D. Giri For Reference 1. Email ID : [email protected] 2. Website : www.dvcgoregaon.edu.in 3. Track ID : MHCOGN23059 Encl. : 1. 5 copies (with CDs) of SSR. 2. LOI of the college dated, 12 Nov. 2014. 3. Your letter No. NAAC/WR/GH/MHCOGN23059/ 1st cycle/2014-15 dated 27 March 2015. -

Professor, Department of Mechanical Engineering Dr
1. Name and full correspondence address: Professor Dr. Bhagwan Fatru Jogi I/c Registrar, Associate Dean, OSD as Joint Director Level (Affiliation), Rector (Hostel Sahyagiri) Professor, Department of Mechanical Engineering Dr. Babasaheb Ambedkar Technological University, Lonere-402 103, Dist: Raigad (Maharashtra State), India. 2. Email(s) and contact number(s): E-mail: [email protected], [email protected] Mobile: +91-9421166370, +91-9421253599 3. Date of Birth: 10/06/1976 4. Gender (M/F/T): M 5. Category Gen/SC/ST/OBC: OBC 6. Academic Qualification (Undergraduate Onwards) School/College/Institute % Degree/Marks Year Class Achievement / University Marks March Pune Board S.S.C. (608/700) 86.85 Distn First Rank 1991 March Pune Board H.S.C. (470/600) 78.33 Distn First Rank 1993 Govt. Engg. College, B.E. (1159/1675) July 69.19 Distn Second Rank Karad (Mech. Engg) 1997 M. Tech. Dr. B.A. Technological (1189/1600) January First Rank University, Lonere- 74.31 Distn (Manufacturing 2005 (Gold-Medal) Raigad Engg.) IIT Bombay, Mumbai Ph. D. (7.88 CPI) 28th April 2014 1 Employment Record (starting from present position): University/College Designation Period Dr. Babasaheb Ambedkar Technological I/c Registrar 14/8/2020-till University, Lonere date OSD as Joint Director Dr. Babasaheb Ambedkar Technological 6/9/2018-till Level (Affiliation) University, Lonere date Dr. Babasaheb Ambedkar Technological 6/9/2018 for one Associate Dean University, Lonere year Tribal Development Dr. Babasaheb Ambedkar Technological 27/4/2017 to till Centre University, Lonere date Coordinator/Director Dr. Babasaheb Ambedkar Technological Professor 17/10/2015-till University, Lonere date Dr. -

DIT Aple Sarkar Center Count.Xlsx
1 Raigad ALIBAG AGARSURE 2137 1 0 1 2 Raigad ALIBAG AKSHI 2976 1 0 1 3 Raigad ALIBAG AMBEPUR 5035 2 2 0 4 Raigad ALIBAG AWAS 4072 1 1 0 5 Raigad ALIBAG BAMANGAON 1815 1 1 0 6 Raigad ALIBAG BELKADE 777 1 1 0 7 Raigad ALIBAG BELOSHI 5748 2 2 0 8 Raigad ALIBAG BORGHAR 3614 1 0 1 9 Raigad ALIBAG BORIS 1220 1 0 1 10 Raigad ALIBAG CHARI 1894 1 0 1 11 Raigad ALIBAG CHENDHARE 9148 2 1 1 12 Raigad ALIBAG CHINCHAVALI 2652 1 0 1 13 Raigad ALIBAG CHINCHOTI 3539 1 0 1 14 Raigad ALIBAG CHAUL 10592 3 3 0 15 Raigad ALIBAG DHAVAR 1881 1 0 1 16 Raigad ALIBAG DHOKAWADE 3674 1 1 0 17 Raigad ALIBAG KAMARLE 3753 1 0 1 18 Raigad ALIBAG KAVIR 2434 1 0 1 19 Raigad ALIBAG KHANAV 5196 2 2 0 20 Raigad ALIBAG KHANDALE 4762 1 0 1 21 Raigad ALIBAG KHIDKI 636 1 0 1 22 Raigad ALIBAG KIHIM 4250 1 1 0 23 Raigad ALIBAG KOPROLI 3009 1 1 0 24 Raigad ALIBAG KURDUS 5942 2 0 2 25 Raigad ALIBAG KURKUNDI KOLTEEMBHI 1666 1 1 0 26 Raigad ALIBAG KURUL 5806 2 2 0 27 Raigad ALIBAG KUSUMBALE 4358 1 0 1 28 Raigad ALIBAG MAN(T) ZIRAD 3936 1 0 1 29 Raigad ALIBAG MANKULE 2805 1 1 0 30 Raigad ALIBAG MAPGAON 5307 2 2 0 31 Raigad ALIBAG MILKATKHAR 1475 1 0 1 32 Raigad ALIBAG MULE 862 1 0 1 33 Raigad ALIBAG NAGAON 6430 2 1 1 34 Raigad ALIBAG NARANGI 847 1 0 1 35 Raigad ALIBAG NAVEDAR NAVGAON 3962 1 0 1 36 Raigad ALIBAG PARHUR 3520 1 0 1 37 Raigad ALIBAG PEDHAMBE 1777 1 0 1 38 Raigad ALIBAG PEZARI 2114 1 0 1 39 Raigad ALIBAG POYNAD 4172 1 1 0 40 Raigad ALIBAG RAMRAJ 4614 1 1 0 41 Raigad ALIBAG RANJANKHAR DAWALI 1419 1 0 1 42 Raigad ALIBAG REVDANDA 9268 2 2 0 43 Raigad ALIBAG REWAS 1654 1 0 1 -

Pincode Officename Mumbai G.P.O. Bazargate S.O M.P.T. S.O Stock
pincode officename districtname statename 400001 Mumbai G.P.O. Mumbai MAHARASHTRA 400001 Bazargate S.O Mumbai MAHARASHTRA 400001 M.P.T. S.O Mumbai MAHARASHTRA 400001 Stock Exchange S.O Mumbai MAHARASHTRA 400001 Tajmahal S.O Mumbai MAHARASHTRA 400001 Town Hall S.O (Mumbai) Mumbai MAHARASHTRA 400002 Kalbadevi H.O Mumbai MAHARASHTRA 400002 S. C. Court S.O Mumbai MAHARASHTRA 400002 Thakurdwar S.O Mumbai MAHARASHTRA 400003 B.P.Lane S.O Mumbai MAHARASHTRA 400003 Mandvi S.O (Mumbai) Mumbai MAHARASHTRA 400003 Masjid S.O Mumbai MAHARASHTRA 400003 Null Bazar S.O Mumbai MAHARASHTRA 400004 Ambewadi S.O (Mumbai) Mumbai MAHARASHTRA 400004 Charni Road S.O Mumbai MAHARASHTRA 400004 Chaupati S.O Mumbai MAHARASHTRA 400004 Girgaon S.O Mumbai MAHARASHTRA 400004 Madhavbaug S.O Mumbai MAHARASHTRA 400004 Opera House S.O Mumbai MAHARASHTRA 400005 Colaba Bazar S.O Mumbai MAHARASHTRA 400005 Asvini S.O Mumbai MAHARASHTRA 400005 Colaba S.O Mumbai MAHARASHTRA 400005 Holiday Camp S.O Mumbai MAHARASHTRA 400005 V.W.T.C. S.O Mumbai MAHARASHTRA 400006 Malabar Hill S.O Mumbai MAHARASHTRA 400007 Bharat Nagar S.O (Mumbai) Mumbai MAHARASHTRA 400007 S V Marg S.O Mumbai MAHARASHTRA 400007 Grant Road S.O Mumbai MAHARASHTRA 400007 N.S.Patkar Marg S.O Mumbai MAHARASHTRA 400007 Tardeo S.O Mumbai MAHARASHTRA 400008 Mumbai Central H.O Mumbai MAHARASHTRA 400008 J.J.Hospital S.O Mumbai MAHARASHTRA 400008 Kamathipura S.O Mumbai MAHARASHTRA 400008 Falkland Road S.O Mumbai MAHARASHTRA 400008 M A Marg S.O Mumbai MAHARASHTRA 400009 Noor Baug S.O Mumbai MAHARASHTRA 400009 Chinchbunder S.O -

Raigad District Aaple Sarkar Seva Kendra List Sr
जे कधारक G2C & B2C चे मळून महयाला ५० Transaction करणार नाहत यांचे सटर तकाळ बंद करणेत येईल. Raigad District Aaple Sarkar Seva Kendra List Sr. Urban/ VLE Name Address Village Taluka Phone email id No. Rural 1 Sonali Sharad Mithe Grampanchyat Office Agarsure Alibag 7066709270 Rural [email protected] 2 Priyanka Chandrakant Naik Grampanchyat Office Akshi Alibag 8237414282 Rural [email protected] Maha-E-Seva Kendra Alibag Court Road Near Tahasil 3 Karuna M Nigavekar Office Alibag Alibag Alibag Alibag 9272362669 urban [email protected] Near Dattapada, Dattanagar, Po. Saral, 4 Neeta Subhash Mokal Alibag Alibag 8446863513 Rural [email protected] Tal. Alibag, Dist. Raigag 5 Shama Sanjay Dongare Grampanchyat Office Ambepur Alibag 8087776107 Rural [email protected] Sarvajanik Suvidha Kendra (Maha E Seva Kendra) Ranjanpada-Zirad 18 Alibag 6 Ashish Prabhakar Mane Awas Alibag 8108389191 Rural [email protected] Revas Road & Internal Prabhat Poultry Road Prabhat Poultry Ranjanpada 7 hemant anant munekar Grampanchyat Office Awas Alibag 9273662199 Rural [email protected] 8 Ashvini Aravind Nagaonkar Grampanchyat Office Bamangaon Alibag 9730098700 Rural [email protected] 262, Rohit E-Com Maha E-Seva Kendra, 9 Sanjeev Shrikant Kantak Belkade Alibag 9579327202 Rural [email protected] Alibag - Roha Road Belkade Po. Kurul 10 Santosh Namdev Nirgude Grampanchyat Office Beloshi Alibag 8983604448 Rural [email protected] Maha E Seva Kendra Bhal 4 Bhal Naka St 11 Shobharaj Dashrath Bhendkar Stand Bhal, -

Multicon-W 2021 Multicon-W
AICTE SPONSORED CONFERENCE Souvenir Technocrat Partners (Gold) IC-TELECON 2021 IC-ICN 2021 IC- AMCE 2021 Print Media Partner t h IC-HSTE 2021 EC 2021 Print Media t h - A platform for Multiple Conferences and Workshops Collaborators IOTW 2021 PBW 2021 Febr uar y 26 & 27 2021, Friday & Saturday Springer LNNS Series th MULTICON-W 2021 MULTICON-W 2021 Dr. B. K. Mishra Programme Chair Autonomous College Affiliated to University of Mumbai Approved by All India Council for Technical Education(AICTE) and Government of Maharashtra A - Block, Thakur Educational Campus, Shyamnarayan Thakur Marg, Thakur Village, Kandivali (East), Mumbai - 400 101 Tel.: 022-6730 8000 / 8106 / 8107 Telefax: 022-2846 1890 • Email: [email protected] • Website: www.tcetmumbai.in • www.thakureducation.org MULTICON-W 2021 HOST INSTITUTE “Transformation To International Level Infrastructure - In Full Swing” th th ISO 9001:2015 NBA NAAC Accredited AICTE-CII Survey rating Among Top 250 68 & 78 in All India Rank by Outlook Certified Accredited Institute in Platinum category for Colleges in NIRF survey published in June 2019 & Institute Programs with 'A' Grade Industry linkages Ranking May 2018 respectively 2021 MULTICON-W 2021 Technocrat Partner Institutes Other Partners Print Media Collaborators Springer LNNS Series Contents 1. Messages 01 2. About Conference 13 3. Outreach of Previous 11 Conferences 25 4. Remembrance of ICWET to Multicon-W 26 5. Glimpses of Conference Publications 38 6. Glimpses of ICWET 2010 to Multicon-W 2019 & Workshops 39 7. Eminent Personalities Visited the Institute 52 8. Thakur Education Group 53 9. Host Institute 55 10. Technocrat Partner Institutes, Other Partners & Collaborators 56 11. -

Public Works Department Government of Maharashtra
Public Works Department Government of Maharashtra INTERNATIONAL COMPETITVE BIDDING UNDER SINGLE STAGE BIDDING PROCESS FOR [HAM-ML 1 - Improvement of roads to Two Laning/Two laning with paved shoulders Under MRIP Package ML-1 (Length of Road 394.39 Km) on Hybrid Annuity Basis in the State of Maharashtra ] REQUEST FOR PROPOSAL (RFP) Volume I INSTRUCTIONS TO BIDDERS Public works Division, Alibag [Date of Issue: 06/07/2018] Table of Contents SECTION 1 16 INTRODUCTION 16 1.1. Background 16 1.2. Brief description of Bidding Process 19 1.3. Schedule of Bidding Process 21 SECTION-2 23 INSTRUCTIONS TO BIDDERS 23 2.1. General terms of Bidding 23 2.2. Eligibility and qualification requirements of Bidder 29 2.3. Proprietary data 36 2.4. Cost of Bidding 36 2.5. Site visit and verification of information 36 2.6. Verification and Disqualification 37 B. DOCUMENTS 40 2.7. Contents of the RFP 40 2.8. Clarifications 40 2.9. Amendment of RFP 41 2.10. Format and Signing of Bid 42 2.11. Documents comprising Technical and Financial Bid 42 2.12. Bid Due Date 45 2.13. Late Bids 45 2.14. Procedure for e-tendering 45 2.15. Online Opening of Bids 47 2.16. Rejection of Bids 47 2.17. Validity of Bids 47 2.18. Confidentiality 48 2.19. Correspondence with the Bidder 48 D. BID SECURITY 49 2.20. Bid Security 49 SECTION-3 51 EVALUATION OF TECHNICAL BIDS AND OPENING & EVALUATION OF FINANCIAL BIDS 51 3.1. Opening and Evaluation of Technical Bids 51 3.2. -

Environmentally Sound Management in Chemical Industries
Environmentally Sound Management in Chemical Industries by Dr.Y.B.Sontakke Regional Officer(HQ)P. K. Mirashe I/c Hazardous SubstancesAsst Management Secretary (Technical) 1 Div.Maharashtra Pollution Control Board 22nd April, 2014 Enforcement of Environmental Legislation by MPCB Water (P&CP) Act, 1974 Air (P&CP) Act, 1981 Water (P&CP) Cess Act, 1977 Environment (Protection) Act, 1986 Hazardous Wastes (M&H) Rules, 1989 & Amended 2003 Hazardous Chemical Rules, 1989 Coastal Regulation Zone Notification, 1991 Municipal Solid Waste Rules, 1998 Bio Medical Waste Rules, 1999 Maharashtra Chemical Industry MIDC LSI & MSI Total no. MIDC LSI & Total no. % ge Of Units MIDC LSI & Total no. MSI % Of Units Jalgaon 22% 112 MSI Of Units ge Nashik 10% 175 % ge Nagpur Tarapur 62% 867 Amravati 100% 100 Dombivali 65% 464 Akola New Bombay 48% 470 Yawatmal Ambernath 61% 662 Taloja 52% 545 Patalganga 55% 210 MIDC LSI & Total no. MSI Of Units % ge Roha 48% 310 MIDC LSI & Total no. Mahad 54% 465 MSI % Of Units Lote Parshuram 64% 270 Ratnagiri 100% 47 ge Kurkumbh 23% 35 Aurangabad 46% 335 Solapur 44% 245 Kolhapur 80% 247 Background There are around 5400 chemical industries in the state of Maharashtra out of these 1043 fall in large scale category. The SSI and MSI units can’t afford to have full-fledged treatment system for effluent & HW. The Govt has facilitate by providing CETP & CHWTSDF for the treatment of effluent & HW generated from these units. The Govt has developed different zone in Maharashtra for sitting of Chemical industry. So as to tackle the environmental issues effectively.