Proteaceae Floral Crops; Cultivar Development and Underexploited Uses
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Macadamia Integrifolia HAES 741)
bioRxiv preprint doi: https://doi.org/10.1101/2020.05.25.114009; this version posted May 27, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Chromosome-scale assembly and annotation of the macadamia genome (Macadamia integrifolia HAES 741) Catherine J. Nock1†*, Abdul Baten1,2†, Ramil Mauleon1, Kirsty S. Langdon1, Bruce Topp3, Craig Hardner3, Agnelo Furtado3, Robert J. Henry3 and Graham J. King1 1Southern Cross Plant Science, Southern Cross University, Lismore NSW 2480, Australia 2AgResearch NZ, Grasslands Research Centre, Palmerston North 4442, New Zealand 3Queensland Alliance for Agriculture and Food Innovation, University of Queensland, St Lucia QLD 4069, Australia †These authors contributed equally to this work *Corresponding author, [email protected] ORCID 0000-0001-5609-4681 Abstract Macadamia integrifolia is a representative of the large basal eudicot family Proteaceae and the main progenitor species of the Australian native nut crop macadamia. Since its commercialisation in Hawaii fewer than 100 years ago, global production has expanded rapidly. However, genomic resources are limited in comparison to other horticultural crops. The first draft assembly of M. integrifolia had good coverage of the functional gene space but its high fragmentation has restricted its use in comparative genomics and association studies. Here we have generated an improved assembly of cultivar HAES 741 (4,094 scaffolds, 745 Mb, N50 413 kb) using a combination of Illumina paired and PacBio long read sequences. -

Method to Estimate Dry-Kiln Schedules and Species Groupings: Tropical and Temperate Hardwoods
United States Department of Agriculture Method to Estimate Forest Service Forest Dry-Kiln Schedules Products Laboratory Research and Species Groupings Paper FPL–RP–548 Tropical and Temperate Hardwoods William T. Simpson Abstract Contents Dry-kiln schedules have been developed for many wood Page species. However, one problem is that many, especially tropical species, have no recommended schedule. Another Introduction................................................................1 problem in drying tropical species is the lack of a way to Estimation of Kiln Schedules.........................................1 group them when it is impractical to fill a kiln with a single Background .............................................................1 species. This report investigates the possibility of estimating kiln schedules and grouping species for drying using basic Related Research...................................................1 specific gravity as the primary variable for prediction and grouping. In this study, kiln schedules were estimated by Current Kiln Schedules ..........................................1 establishing least squares relationships between schedule Method of Schedule Estimation...................................2 parameters and basic specific gravity. These relationships were then applied to estimate schedules for 3,237 species Estimation of Initial Conditions ..............................2 from Africa, Asia and Oceana, and Latin America. Nine drying groups were established, based on intervals of specific Estimation -

PUBLISHER S Candolle Herbarium
Guide ERBARIUM H Candolle Herbarium Pamela Burns-Balogh ANDOLLE C Jardin Botanique, Geneva AIDC PUBLISHERP U R L 1 5H E R S S BRILLB RI LL Candolle Herbarium Jardin Botanique, Geneva Pamela Burns-Balogh Guide to the microform collection IDC number 800/2 M IDC1993 Compiler's Note The microfiche address, e.g. 120/13, refers to the fiche number and secondly to the individual photograph on each fiche arranged from left to right and from the top to the bottom row. Pamela Burns-Balogh Publisher's Note The microfiche publication of the Candolle Herbarium serves a dual purpose: the unique original plants are preserved for the future, and copies can be made available easily and cheaply for distribution to scholars and scientific institutes all over the world. The complete collection is available on 2842 microfiche (positive silver halide). The order number is 800/2. For prices of the complete collection or individual parts, please write to IDC Microform Publishers, P.O. Box 11205, 2301 EE Leiden, The Netherlands. THE DECANDOLLEPRODROMI HERBARIUM ALPHABETICAL INDEX Taxon Fiche Taxon Fiche Number Number -A- Acacia floribunda 421/2-3 Acacia glauca 424/14-15 Abatia sp. 213/18 Acacia guadalupensis 423/23 Abelia triflora 679/4 Acacia guianensis 422/5 Ablania guianensis 218/5 Acacia guilandinae 424/4 Abronia arenaria 2215/6-7 Acacia gummifera 421/15 Abroniamellifera 2215/5 Acacia haematomma 421/23 Abronia umbellata 221.5/3-4 Acacia haematoxylon 423/11 Abrotanella emarginata 1035/2 Acaciahastulata 418/5 Abrus precatorius 403/14 Acacia hebeclada 423/2-3 Acacia abietina 420/16 Acacia heterophylla 419/17-19 Acacia acanthocarpa 423/16-17 Acaciahispidissima 421/22 Acacia alata 418/3 Acacia hispidula 419/2 Acacia albida 422/17 Acacia horrida 422/18-20 Acacia amara 425/11 Acacia in....? 423/24 Acacia amoena 419/20 Acacia intertexta 421/9 Acacia anceps 419/5 Acacia julibross. -

PROTEACEAE – It's All About Pollination
PROTEACEAE – it’s all about pollination …….Gail Slykhuis Illustration Philippa Hesterman, images Ellinor Campbell & Marg McDonald A predominantly southern hemisphere plant family, Proteaceae is well represented in Australia, particularly in the West, but we do have our own equally special local representatives, some of which are outlined below. A characteristic feature of many genera within this plant family is the ‘pollen presenter’, which is a fascinating mechanism by which the pollen, which would otherwise be difficult to access for potential pollination vectors such as bees, birds and nectarivorous mammals, is positioned on the extended style of the flower, facilitating cross- pollination. The stigma, which is part of the style, is not mature at this time, thus avoiding self-pollination. A hand lens would enable you to clearly see pollen presenters on the following local representatives: Banksia marginata, Grevillea infecunda, Hakea spp., Isopogon ceratophyllus and Lomatia illicifolia. It is interesting to note that both Victorian Smoke-bush Conospermum mitchellii and Prickly Geebung Persoonia juniperina, also found in our district, do not have pollen presenters. Silver Banksia Banksia marginata This shrub or small tree is readily recognisable when flowering (Feb – July) by the conspicuous yellow pollen presenters, which are an obvious floral part of the banksia flower. These flowers then slowly mature into our iconic woody banksia cones. It is interesting to observe the changes in the nature of the pollen presenters as the flower develops. The white undersides of the leathery leaves provide a clue to the choice of common name with their tip being characteristically blunt or truncate. Anglesea Grevillea Grevillea infecunda One of our endemic plants, the Anglesea Grevillea was first named in 1986 and is Anglesea Grevillea found in several locations north west of Anglesea. -

Cunninghamia Date of Publication: February 2020 a Journal of Plant Ecology for Eastern Australia
Cunninghamia Date of Publication: February 2020 A journal of plant ecology for eastern Australia ISSN 0727- 9620 (print) • ISSN 2200 - 405X (Online) The Australian paintings of Marianne North, 1880–1881: landscapes ‘doomed shortly to disappear’ John Leslie Dowe Australian Tropical Herbarium, James Cook University, Smithfield, Qld 4878 AUSTRALIA. [email protected] Abstract: The 80 paintings of Australian flora, fauna and landscapes by English artist Marianne North (1830-1890), completed during her travels in 1880–1881, provide a record of the Australian environment rarely presented by artists at that time. In the words of her mentor Sir Joseph Dalton Hooker, director of Kew Gardens, North’s objective was to capture landscapes that were ‘doomed shortly to disappear before the axe and the forest fires, the plough and the flock, or the ever advancing settler or colonist’. In addition to her paintings, North wrote books recollecting her travels, in which she presented her observations and explained the relevance of her paintings, within the principles of a ‘Darwinian vision,’ and inevitable and rapid environmental change. By examining her paintings and writings together, North’s works provide a documented narrative of the state of the Australian environment in the late nineteenth- century, filtered through the themes of personal botanical discovery, colonial expansion and British imperialism. Cunninghamia (2020) 20: 001–033 doi: 10.7751/cunninghamia.2020.20.001 Cunninghamia: a journal of plant ecology for eastern Australia © 2020 Royal Botanic Gardens and Domain Trust www.rbgsyd.nsw.gov.au/science/Scientific_publications/cunninghamia 2 Cunninghamia 20: 2020 John Dowe, Australian paintings of Marianne North, 1880–1881 Introduction The Marianne North Gallery in the Royal Botanic Gardens Kew houses 832 oil paintings which Marianne North (b. -

South Africa Cape Wildflowers, Birding & Big Game II 21St August to 3Rd September 2022 (14 Days)
South Africa Cape Wildflowers, Birding & Big Game II 21st August to 3rd September 2022 (14 days) Cape Mountain Zebras & wildflowers in West Coast NP by Adam Riley This comprehensive tour covers the most exciting regions of the Cape in our quest to experience both breathtaking displays of wildflowers and to track down some of the country’s endemic birds. We begin in the vibrant city of Cape Town, where Table Mountain provides a spectacular backdrop to the immensely diverse fynbos that cloaks the cities periphery. This fynbos constitutes the Cape Floral Kingdom – the smallest and richest of the world’s 6 floral kingdoms. It is also the only floral kingdom to be confined to the boundaries of a single country. Thereafter we venture to the West Coast and Namaqualand, which boast an outrageous and world famous floral display in years of good rains, before travelling through the heart of the country’s semi-desert region, focusing on the special bird’s endemic to this ancient landscape. We conclude the journey heading out of wildflower country to Augrabies Falls, an area offering unparalleled raptor viewing and a wide range of dry region birds. We invite you on this celebration of some of the finest wildflower and endemic birding that the African continent has to offer! RBT South Africa - Cape Wildflowers, Birding & Big Game 2 THE TOUR AT A GLANCE… THE ITINERARY Day 1 Arrival in Upington Day 2 Upington to Augrabies Falls National Park Day 3 Augrabies Falls National Park Day 4 Augrabies Falls National Park to Springbok Day 5 Springbok to Nieuwoudtville -

Proteaceae), with a Key to the Species of Phaeophleospora
Fungal Diversity Phaeophleospora faureae comb. novo associated with leaf spots on Faurea saligna (Proteaceae), with a key to the species of Phaeophleospora Joanne E. Taylor* and Pedro W. Crous Department of Plant Pathology, University of Stellenbosch, Private Bag Xl, Stellenbosch 7602, South Africa; * e-mail: [email protected] Taylor, J.E. and erous, P.W. (1999). Phaeophleosporafaureae comb. novo associated with leaf spots on Faurea saligna (Proteaceae), with a key to the species of Phaeophleospora. Fungal Diversity 3: 153-158. During studies of the fungal pathogens occurring on Proteaceae in South Africa, the type specimen of Stilbospora faureae was examined. This fungus was found to be a species of Phaeophleospora, and is transferred to this genus in the present paper. A key to the species in Phaeophleospora is also given. Key words: pathogen, Phaeophleospora, Proteaceae, Stilbospora Introduction Phaeophleospora was considered to be a nomen dubium (Sutton, 1977), until Crous et al. (1997) resurrected it as an earlier name for the coelomycete genus Kirramyces 1. Walker, B. Sutton and 1. Pascoe. There are currently 11 species in Phaeophleospora (Walker et al., 1992; Sutton, 1993; Palm, 1996; Wingfield et al., 1996; Wu et al., 1996; Crous et al., 1997; Crous, 1998; Crous and Palm, 1999) and three of these occur on Proteaceae hosts. Phaeophleospora is associated with leaf spots and is characterised by sub• epidermal, dark-walled pycnidia, which become open and cup-shaped at maturity (Crous et al., 1997). Under conditions of high humidity, these conidiomata exude masses of conidia in a long, brown to black cirrus (Crous et al., 1997). -

IV. on the Proteaceć of Jussieu. by Mr. Robert Brown, Lib. LS
IV. On the Proteacea of Jussieu. By -Mr. Robert Brown, Lib. L.S. Read Jan. 17, 1809. THELinnean system of botany, though confessedly artificial, has not only contributed more than all others to facilitate tlie knowledge of species, but, by constantly directing the attention to those essential parts of the flower on which it is founded, has made us acquainted with more of their important modific-a t’ ions than we probably should have known, had it not been generally adopted, and has thus laid a more solid foundation for the esta- blishment of a natural arrangement, the superior importance of which no one has been inore fully impressed with than Linnzus hiinself. There are still, however, certain circumstances respccting the stamina and pistilla, which appear to iiie to havc been much less attended to than they deserve, both by Linneus and succeeding botanists. What I chiefly allude to is the state of these organs before the expansion of the flower. Tlie utility of ascertaining the internal condition of the ovarium before fecundation will liardly be called in question, now that the immortal worlis of Gxrtner and Jussieu hare demonstrated the necessity of minutely studying the fruits of plants in attempting to arrange tlicin ac- cording to tlic sum of their affinities, as in many cases the true nature of tlie ripc fruit, cspecially witli respect to the placenta- tion of the seeds, can oiily be detcrniined by this mc;~ns. Its importance is indeed expressly inculcated by many l~ot:inists, Tf’llO, 16 Mr. BROWN,on the Proteacee of Jussieu. -
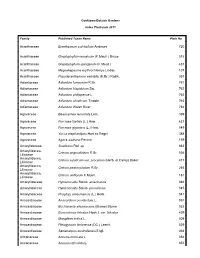
Abelmoschus Moschatus Subsp
Cooktown Botanic Gardens Index Plantarum 2011 Family Published Taxon Name Plate No Acanthaceae Eranthemum pulchellum Andrews 720 Acanthaceae Graptophyllum excelsum (F.Meull.) Druce 515 Acanthaceae Graptophyllum spinigerum (F.Meull.) 437 Acanthaceae Megaskepasma erythrochlamys Lindau 107 Acanthaceae Pseuderanthemum variabile (R.Br.) Radlk. 357 Adiantaceae Adiantum formosum R.Br. 761 Adiantaceae Adiantum hispidulum Sw. 762 Adiantaceae Adiantum philippense L. 765 Adiantaceae Adiantum silvaticum Tindale 763 Adiantaceae Adiantum Walsh River 764 Agavaceae Beaucarnea recurvata Lem. 399 Agavaceae Furcraea foetida (L.) Haw. 637 Agavaceae Furcraea gigantea (L.) Haw. 049 Agavaceae Yucca elephantipes Hort.ex Regel 388 Agavaceae Agave sisalana Perrine. 159 Amarylidaceae Scadoxus Raf. sp 663 Amaryllidacea, Crinum angustifolium R.Br. 536 Liliaceae Amaryllidacea, Crinum asiaticum var. procerum (Herb. et Carey) Baker 417 Liliaceae Amaryllidacea, Crinum pedunculatum R.Br. 265 Liliaceae Amaryllidacea, Crinum uniflorum F.Muell. 161 Liliaceae Amaryllidaceae Hymenocallis Salisb. americanus 046 Amaryllidaceae Hymenocallis Salisb. peruvianna 045 Amaryllidaceae Proiphys amboinensis (L.) Herb. 041 Anacardiaceae Anacardium occidentale L. 051 Anacardiaceae Buchanania arborescens (Blume) Blume. 022 Anacardiaceae Euroschinus falcatus Hook.f. var. falcatus 429 Anacardiaceae Mangifera indica L. 009 Anacardiaceae Pleiogynium timorense (DC.) Leenh. 029 Anacardiaceae Semecarpus australiensis Engl. 368 Annonaceae Annona muricata L. 054 Annonaceae Annona reticulata L. 053 Annonaceae Annona squamosa 602 Annonaceae Cananga odorata (Lam.) Hook.f.&Thomson 406 Annonaceae Melodorum leichhardtii (F.Muell.) Diels. 360 Annonaceae Rollinia deliciosa Saff. 098 Apiaceae Centella asiatica (L.) Urb. 570 Apocynaceae Adenium obesum (Forssk.) Roem. & Schult. 489 Apocynaceae Allamanda cathartica L. 047 Apocynaceae Allamanda violacea Gardn. & Field. 048 Apocynaceae Alstonia actinophylla (A.Cunn.) K.Schum. 026 Apocynaceae Alstonia scholaris (L.) R.Br. 012 Apocynaceae Alyxia ruscifolia R.Br. -

Museum of Economic Botany, Kew. Specimens Distributed 1901 - 1990
Museum of Economic Botany, Kew. Specimens distributed 1901 - 1990 Page 1 - https://biodiversitylibrary.org/page/57407494 15 July 1901 Dr T Johnson FLS, Science and Art Museum, Dublin Two cases containing the following:- Ackd 20.7.01 1. Wood of Chloroxylon swietenia, Godaveri (2 pieces) Paris Exibition 1900 2. Wood of Chloroxylon swietenia, Godaveri (2 pieces) Paris Exibition 1900 3. Wood of Melia indica, Anantapur, Paris Exhibition 1900 4. Wood of Anogeissus acuminata, Ganjam, Paris Exhibition 1900 5. Wood of Xylia dolabriformis, Godaveri, Paris Exhibition 1900 6. Wood of Pterocarpus Marsupium, Kistna, Paris Exhibition 1900 7. Wood of Lagerstremia parviflora, Godaveri, Paris Exhibition 1900 8. Wood of Anogeissus latifolia , Godaveri, Paris Exhibition 1900 9. Wood of Gyrocarpus jacquini, Kistna, Paris Exhibition 1900 10. Wood of Acrocarpus fraxinifolium, Nilgiris, Paris Exhibition 1900 11. Wood of Ulmus integrifolia, Nilgiris, Paris Exhibition 1900 12. Wood of Phyllanthus emblica, Assam, Paris Exhibition 1900 13. Wood of Adina cordifolia, Godaveri, Paris Exhibition 1900 14. Wood of Melia indica, Anantapur, Paris Exhibition 1900 15. Wood of Cedrela toona, Nilgiris, Paris Exhibition 1900 16. Wood of Premna bengalensis, Assam, Paris Exhibition 1900 17. Wood of Artocarpus chaplasha, Assam, Paris Exhibition 1900 18. Wood of Artocarpus integrifolia, Nilgiris, Paris Exhibition 1900 19. Wood of Ulmus wallichiana, N. India, Paris Exhibition 1900 20. Wood of Diospyros kurzii , India, Paris Exhibition 1900 21. Wood of Hardwickia binata, Kistna, Paris Exhibition 1900 22. Flowers of Heterotheca inuloides, Mexico, Paris Exhibition 1900 23. Leaves of Datura Stramonium, Paris Exhibition 1900 24. Plant of Mentha viridis, Paris Exhibition 1900 25. Plant of Monsonia ovata, S. -

Finschia-"A Genus of "Nut" Trees of the Southwest Pacific
Finschia-" A Genus of "Nut" Trees of the Southwest Pacific c. T. WHITE1 INTRODUCTION A PLANT FAMILY with a most interesting and F. Muell., Carnarvonia F. Muell., D arlin"gia F; intriguing distribution is Proteaceae, which finds Muell., Hollandaea F. Muell. (two spp.) , Mus its greatest development in Australia (650 " gravea F. Muell., and Placospermum White & species) on the one hand and South Africa (300 Francis. A surprising feature is the absence, species) on the other, though the two countries with the exception of one species in New Zea have no genera in common. Practically all the land, of the family "from Polynesia. South African species and the vast majority of There is in the islands of the southwest Paci "Australian ones are markedly xerophytic. The fic-Caroline Islands, New Guinea, Solomon largest genus, Greoillea R. Br., consists mainly Islands, and the New Hebrides-a group of trees of xerophytic shrubs or small trees but a few with the floral characters of Greuillea R. Br. are large trees found in the rain forests of and the fruit of Helicia Lour. These, I consider, tropical and subtropical eastern Australia, New all belong 'to Finschia Warb. This genus was Guinea, and New Caledonia. In the southwest founded by Warburg (1891: 297 ) on"a tree Pacific area the family finds its greatest develop from northeastern New Guinea. His original ment in northeastern Australia, where trees be description would cover Grevillea R. Br. exactly longing to it provide the great bulk of cabinet though he does not mention this genus and on timbers known in the trade as "Silky Oaks." the following page the distinctions he gives for There is close affinity between the Proteaceae of separating his proposed new genus from H elicia eastern Australia and of western South America are exactly those which distinguish Greuillea as illustrated by the genera Embothrium Forst. -

Protea May Predict Occurrence and Abundance Alan T.K
Cape Sugarbird: Flowering Protea may predict occurrence and abundance Alan T.K. Lee 1, 2 1 Birds & Environmental Change Programme, Climate Change & BioAdaptation Division, South African National Biodiversity Institute 2 Percy FitzPatrick Institute of African Ornithology, University of Cape Town, Rondebosch 7701, South Africa [email protected] Introduction Results The Cape Sugarbird Promerops cafer (Fig. 1) is one of six 1.A total of 361 Cape Sugarbirds (249 groups) were recorded species of bird restricted to the Fynbos biome. The bird is an during 820 counts, with an overall density estimate of 17 (13-21) important pollinator of certain Protea species and the close birds/km2 association between this endemic bird and a subgroup of flowering species of the Protea genus has been known for 2.17% of points were classified as ‘proteoid’, and 55% of bird some time 1. Reliance of Cape Sugarbirds on other Fynbos encounters were recorded in this habitat type (Fig. 3) families or environmental variables less well known. 3.Cape Sugarbird abundance was strongly positively correlated Increased fire frequency and climate change may impact on with bird pollinated Protea, Protea flower scores, vegetation this species habitat 2, and some evidence suggests range height and other proteaceae; while negatively correlated with has contracted over the last 20 years 3. Loss of this key grass, rocky outcrop and plant families associated with dry species may have implications for the pollination of a wide Fynbos (Table 1) range of Protea species. We aimed to: 1.Determine the status of Cape Sugarbird across the Fynbos biome; 2.