Hemp-Based Products Intended for Human Consumption (Cod
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Dispensary Selection Information
______________________________________________________________________________________ State of Vermont Department of Public Safety Marijuana Registry [phone] 802-241-5115 45 State Drive [fax] 802-241-5230 Waterbury, Vermont 05671-1300 [email] [email protected] www.medicalmarijuana.vermont.gov Dispensary Selection Information IMPORTANT INFORMATION CONTAINED BELOW WHEN COMPLETING THE FORMS TO REGISTER AS A PATIENT WITH THE VERMONT MARIJUANA REGISTRY (VMR). Materials provided by each dispensary are attached to assist patient applicants designating a dispensary. Registered patients may purchase marijuana, marijuana infused products, seeds, and clones from a registered dispensary. Registered patients who designate a dispensary may purchase marijuana products and cultivate marijuana in a single secure indoor facility. Registered patients who elect to cultivate marijuana in a single secure indoor facility must provide the address and location to the VMR on his or her application. Any updates to the address and/or location of the single secure indoor facility must be submitted in writing via email or mail to the contact information below. Registered patients may only purchase marijuana, marijuana infused products, seeds, and clones from one dispensary and must designate the dispensary of his or her choice on their application. Registered patients may only change dispensaries every 30 days. After 30 days, a registered patient may change his or her designated dispensary by submitting a completed Cardholder Information Notification form and a $25 processing fee. The VMR will issue the registered patient a new registry identification card with a new registry identification number. ALL registered patients and caregivers MUST schedule appointments prior to going to their designated dispensary to obtain marijuana, this includes seed and clones. -

Big-Catalogue-English-2020.Pdf
PAS CH SIO UT N D ® CATALOGUE English SEED COMPANY Feminized, autoflower and regular cannabis seeds AMSTERDAM, ESTABLISHED 1987 for recreational and medical use. Amsterdam - Maastricht YOUR PASSION OUR PASSION DUTCH PASSION 02 Contents Welcome to Dutch Passion Welcome to Dutch Passion 02 Dutch Passion was the second Cannabis Seed Company in the world, established in Amsterdam in 1987. It is our mission to supply Bestsellers 2019 02 the recreational and medical home grower with the highest quality cannabis products available in all countries where this is legally Regular, Feminized and Autoflower 03 allowed. Cannabinoids 03 Medical use of cannabis 03 After many years of dedication Dutch Passion remains a leading supplier of the world’s best cannabis genetics. Our experienced Super Sativa Seed Club 04 team do their utmost to maintain the quality of our existing varieties and constantly search for new ones from an extensive network Special Cannabinoids / THC-Victory 05 of worldwide sources. We supply thousands of retailers and seed distributors around the world. Dutch Outdoor 06 High Altitude 09 CBD Rich 10 Dutch Passion have never been afraid to upset conventional thinking; we invented feminized seeds in the 1990’s and more recently Latin America 13 have pioneered the introduction of 10-week Autoflower seeds which have helped make life even easier for the self-sufficient Classics 14 cannabis grower. CBD-rich medical cannabis genetics is a new area that we are proud to be leading. Skunk Family 19 Orange Family 21 The foundation of our success is the genetic control we have over our strains and the constant influx of new genetics that we obtain Blue Family 24 worldwide. -

A Multifaceted Approach to Address Variation in Cannabis Sativa
University of Northern Colorado Scholarship & Creative Works @ Digital UNC Dissertations Student Research 5-2019 A Multifaceted Approach to Address Variation in Cannabis Sativa Anna Louise Schwabe Follow this and additional works at: https://digscholarship.unco.edu/dissertations Recommended Citation Schwabe, Anna Louise, "A Multifaceted Approach to Address Variation in Cannabis Sativa" (2019). Dissertations. 554. https://digscholarship.unco.edu/dissertations/554 This Text is brought to you for free and open access by the Student Research at Scholarship & Creative Works @ Digital UNC. It has been accepted for inclusion in Dissertations by an authorized administrator of Scholarship & Creative Works @ Digital UNC. For more information, please contact [email protected]. © 2019 ANNA LOUISE SCHWABE ALL RIGHTS RESERVED UNIVERSITY OF NORTHERN COLORADO Greeley, Colorado The Graduate School A MULTIFACETED APPROACH TO ADDRESS VARIATION IN CANNABIS SATIVA A Dissertation Submitted in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy Anna Louise Schwabe College of Natural and Health Sciences School of Biological Sciences Biological Education May 2019 This Dissertation by: Anna Louise Schwabe Entitled: A Multifaceted Approach to Address Variation in Cannabis sativa has been approved as meeting the requirement for the Degree of Doctor of Philosophy in College of Natural and Health Sciences in School of Biological Sciences, Program of Biological Education. Accepted by the Doctoral Committee ____________________________________________________ -

ARMENIA: COUNTRY REPORT to the FAO INTERNATIONAL TECHNICAL CONFERENCE on PLANT GENETIC RESOURCES (Leipzig,1996)
ARMENIA: COUNTRY REPORT TO THE FAO INTERNATIONAL TECHNICAL CONFERENCE ON PLANT GENETIC RESOURCES (Leipzig,1996) Prepared by: Ministry of Agriculture Yerevan, June 1995 ARMENIA country report 2 Note by FAO This Country Report has been prepared by the national authorities in the context of the preparatory process for the FAO International Technical Conference on Plant Genetic Resources, Leipzig, Germany, 17-23 June 1996. The Report is being made available by FAO as requested by the International Technical Conference. However, the report is solely the responsibility of the national authorities. The information in this report has not been verified by FAO, and the opinions expressed do not necessarily represent the views or policy of FAO. The designations employed and the presentation of the material and maps in this document do not imply the expression of any option whatsoever on the part of the Food and Agriculture Organization of the United Nations concerning the legal status of any country, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. ARMENIA country report 3 Table of contents CHAPTER 1 INTRODUCTION TO THE REPUBLIC OF ARMENIA AND ITS AGRICULTURAL SECTOR 4 1.1 MAJOR TYPES OF FORESTS 6 1.2 AGRICULTURAL SECTOR 6 CHAPTER 2 INDIGENOUS PLANT GENETIC RESOURCES 8 2.1 FOREST GENETIC RESOURCES 8 2.2 CEREALS 10 2.3 GRAIN LEGUMES 12 2.4 FORAGE GRASSES 12 2.5 FRUIT AND BERRY PLANTS 13 2.6 VEGETABLES AND MELONS 14 2.7 WILD EDIBLE PLANTS 14 CHAPTER 3 NATIONAL EFFORTS IN PLANT GENETIC RESOURCES CONSERVATION -

Strain Collection Catalogue
STRAIN COLLECTION catalogue WWW.FEMALESEEDS.NL Index INDOOR Blueberry Cheesecake 6 Bubblegummer 7 Lemon Kush 8 Pure AK 9 Skunk Special 10 White Widow x Big Bud 11 ICE 12 Iced Grapefruit 13 C99 14 Grapefruit 16 Sex Bud 17 OUTDOOR Easy Sativa 20 Red Purps 21 Purple Maroc 22 Maroc 23 AUTOMATIC FLOWERING STRAINS Auto AK 26 Auto Bubble 27 Auto Kush 28 Auto Speed Bud 29 Auto WW x BB 30 Auto NL 32 Auto Haze 33 Strain advices 34 Indoor These Female Seeds varieties are not only tasty but also a pleasure for your mind. Most of them are very high and energetic so you will not only get wonderful and great ideas, but also enough energy to do something and make full use of your creativity. Enjoy! Here you find our current tasty indoor selection. Blueberry BubbleGummer Cheesecake Height: 60 - 80 cm Height: 80 - 100 cm Flowering time: 8 weeks Flowering time: 7-8 weeks Seed to Harvest: 11 Seed to harvest: 10 - 11 weeks weeks Harvest: medium Harvest: high quality, high Taste/Smell: sweet and yield harvest fruity bubblegum odor and Taste/Smell: Extremely taste. strong, pungent creosote Effect: Euphoric, smooth aroma followed by an unusual and soft, perfect for getting cheesecake smell. things done during the day. Effect: Powerful stoned, whilst the body is in balance and relaxed. The BubbleGum cannabis was originally developed by growers in Indiana, USA. Our Blueberry Cheesecake From there the genetics (Cheese x Blueberry multiple moved to New England and hybrid) is selected from an extremely strong, smelly mother (you eventually Holland. -

A Short History and Use of Hemp
Agricultura no. 3 - 4 (115-116)/2020 Agriculture A SHORT HISTORY AND USE OF HEMP GÂDEA Ştefania, Ileana BOGDAN, Anamaria VÂTCĂ, Valentina STOIAN, Sorin VÂTCĂ* University of Agricultural Sciences and Veterinary Medicine Cluj-Napoca, 3-5 Calea Mănăştur Street, 400372, Cluj-Napoca, Romania *Corresponding author: [email protected] Abstract. The possibilities of using the hemp plant are endless. A wide range of products can be obtained from this plant, from clothing to paper, from crockery to automotive components and from musical instruments to bird feed. It is known that this plant fits well in almost any climatic conditions, grows quickly and needs very little maintenance. It requires no chemicals and is resistant to pests and diseases. Hemp uses very little water per kilogram of fiber compared to cotton and stores CO2 during growth, which increases its photosynthetic efficiency. In addition, it improves the soil, being ideal for crop rotation. Keywords: cannabis, cannabinoids, psychoactive effect. INTRODUCTION Cannabis is the only plant genus that contains a unique class of molecular compounds, cannabinoids, a family of complex chemicals that act on cannabinoid receptors, which are protein molecules located on the surface of cells (Andre et al., 2016) so called nutraceuticals (Hartsel et al., 2016). Researchers have discovered several types of cannabinoids, the most important of which are delta-9 tetrahydrocannabinol (THC), a psychoactive compound and cannabidiol (CBD) (Grotenhermen, 2003), an anti-psychoactive compound (Clarke and Merlin, 2016; Baron, 2015). It is known that humans have two types of cannabinoid receptors, CB1 and CB2, which are found in different places and have different functions. -

Com(2010)0539
EN EN EN EUROPEAN COMMISSION Brussels, 30.9.2010 COM(2010) 539 final 2010/0267 (COD) Proposal for a REGULATION OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL amending Council Regulation (EC) No 73/2009 establishing common rules for direct support schemes for farmers under the common agricultural policy and establishing certain support schemes for farmers EN EN EXPLANATORY MEMORANDUM 1. CONTEXT OF THE PROPOSAL • Grounds for and objectives of the proposal To align The Commission implementing powers in Council Regulation (EC) No 73/20091 to the differentiation between delegated and implementing powers of the Commission introduced by Articles 290 and 291 of the Treaty on the Functioning of the European Union (TFUE). • General context Articles 290 and 291 of the Treaty on the Functioning of the European Union (TFUE) distinguish two different types of Commission acts: – Article 290 of the TFUE allows the legislator to delegate to the Commission the power to adopt non-legislative acts of general application to supplement or amend certain non-essential elements of a legislative act. Legal acts adopted by the Commission in this way are referred to in the terminology used by the Treaty as "delegated acts" (Article 290(3)). – Article 291 of the TFUE allows Member States to adopt all measures of national law necessary to implement legally binding Union acts. Those acts can confer implementing powers on the Commission where uniform conditions for implementing them are needed. Legal acts adopted by the Commission in this way are referred to in the terminology used by the Treaty as "implementing acts" (Article 291(4)) • Existing provisions in the area of the proposal Articles 290 and 291 of the Treaty on the Functioning of the European Union (TFUE). -
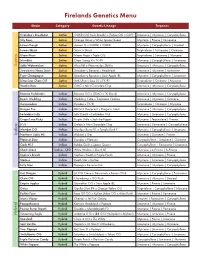
Firelands Genetics Menu
Firelands Genetics Menu Strain Category Genetic Lineage Terpenes Grandpa’s Breathstar Sativa OGKB (OG Kush Breath) x (Tahoe OG x GDP) Limonene | Myrcene | Caryophyllene Jilly Bean Sativa Orange Velvet x White Space Queen Myrcene | Pinene | Farnesene Lemon Dough Sativa (Lemon G x OGKB) x OGKB Myrcene | Caryophyllene | Linalool Lemon Skunk Sativa Skunk x Skunk Terpinolene | Farnesene | Ocimene Miami Heat Sativa Miami Haze x Triple OG Terpinolene | Limonene | Myrcene Mumbles Sativa Chem Dawg 4 x TK-91 Myrcene | Caryophyllene | Limonene Salty Watermelon Sativa Alien Rift x Watermelon Zkittles Limonene | Myrcene | Caryophyllene Strawberry Nana Stash Sativa Strawberry Banana x Headstash Limonene | Myrcene | Farnesene Tiger Champagne Sativa Strawberry Banana x Sour Apple IBL Myrcene | Caryophyllene | Limonene Ultra Sour Chem OG Sativa (MK Ultra x Sour D) x TK-91 Terpinolene | Ocimene | Myrcene Wonka Bars Sativa GMO x Mint Chocolate Chip Limonene | Myrcene | Caryophyllene Banana Puddintain Indica Banana OG x (GMO x TK Skunk) Limonene | Myrcene | Caryophyllene Beach Wedding Indica Wedding Cake x Tropicana Cookies Limonene | Myrcene | Ocimene Cannaradosi Indica Dosidos x TK-91 Terpinolene | Ocimene | Myrcene Dragon Fire Indica 88 G13 Hashplant x Dragon’s Stash Limonene | Myrcene | Caryophyllene Forbidden Jelly Indica Jelly Breath x Forbidden Fruit Myrcene | Limonene | Caryophyllene Grape Lime Ricky Indica Purple Urkle x Jack the Ripper Myrcene | Terpinolene | Pinene It’s It Indica Gelato x Mint Chocolate Chip Limonene | Farnesene | Caryophyllene -

ARMENIA National Report on the State of Plant Genetic Resources in Armenia
COUNTRY REPORT ON THE STATE OF PLANT GENETIC RESOURCES FOR FOOD AND AGRICULTURE ARMENIA National report on the State of Plant Genetic Resources in Armenia MINISTRY OF AGRICULTURE OF THE REPUBLIC OF ARMENIA Yerevan – 2008 Note by FAO This Country Report has been prepared by the national authorities in the context of the preparatory process for the Second Report on the State of World’s Plant Genetic Resources for Food and Agriculture. The Report is being made available by the Food and Agriculture Organization of the United Nations (FAO) as requested by the Commission on Genetic Resources for Food and Agriculture. However, the report is solely the responsibility of the national authorities. The information in this report has not been verified by FAO, and the opinions expressed do not necessarily represent the views or policy of FAO. The designations employed and the presentation of material in this information product do not imply the expression of any opinion whatsoever on the part of FAO concerning the legal or development status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. The mention of specific companies or products of manufacturers, whether or not these have been patented, does not imply that these have been endorsed or recommended by FAO in preference to others of a similar nature that are not mentioned. The views expressed in this information product are those of the author(s) and do not necessarily reflect the views of FAO. CONTENTS LIST OF ACRONYMS AND ABBREVIATIONS 7 INTRODUCTION 8 1. -

That Which We Call Indica, by Any Other Name Would Smell As Sweet
Cannabinoids 2014;9(1):9-15 Original Article That which we call Indica, by any other name would smell as sweet An essay on the history of the term Indica and the taxonomical conflict between the monotypic and polytypic views of Cannabis Jacob L. Erkelens, Arno Hazekamp Bedrocan BV, The Netherlands This article can be downloaded, printed and distributed freely for any non-commercial purposes, provided the original work is proper- ly cited (see copyright info below). Available online at www.cannabis-med.org Author's address: Arno Hazekamp, [email protected] What’s in a name? this may simply be regarded as an anthropological curiosity, a more fundamental issue exists at the root of this, because over the last few centuries there has been An interesting feature of the worldwide subculture a continuing scientific controversy regarding the taxo- devoted to cannabis is the endless number of names nomic classification of cannabis. given to its preparations (marijuana, pot, weed, kiff, Today, a firm belief is held by growers and users of bhang..). On top of that, there is a continuously grow- cannabis that there exist at least two different main ing list of names used to describe different varieties types of cannabis; sativa and indica. However, over the and strains of the cannabis plant. As a result of centu- centuries opinions have been going back and forth over ries of breeding and selection, a large variation of can- whether cannabis is most accurately classified as one nabis strains has been developed. These are commonly single species or as multiple. The roots of this conflict distinguished, by plant breeders, recreational users, and are mostly found in the nineteenth century with tendrils medical cannabis patients alike, through the use of stretching back in time as far as the Late Middle Ages. -
CSE Marijuana List Showing 178 of 712 Listings
CSE Marijuana List Showing 178 of 712 listings Listing Company Sector Company Description Date 1933 Industries is a Nevada-based, growth-orientated company, focusing on the cultivation and manufacturing of cannabis consumer branded goods in a wide range of product formats. Operating through two subsidiaries, the Company controls all aspects of 1933 Industries US the value chain with cultivation, extraction, processing, 16-Jun-17 Inc. (CSE:TGIF) Cannabis and manufacturing assets supporting its diversified portfolio of cannabis brands and licensing partners. The Company owns 91% of Alternative Medicine Association, LC (AMA), and 100% of Infused MFG LLC. www.1933industries.com ... 4Front Ventures Corp. is a vertically-integrated cannabis company with cultivation, production and retail facilities spread across several states in the US. Share Classes 4Front has two classes of shares: subordinate voting shares (“SVS”) and multiple voting shares (“MVS”). SVS are publicly traded on the CSE and are entitled to one vote. MVS are not publicly traded, 4Front Ventures US can convert to SVS at a ratio of 1 MVS to 1 SVS, and 14-Mar-18 Corp. (CSE:FFNT) Cannabis are entitled to 800 votes per MVS. There are 592,627,966 SVS outstanding and 1,276,208 MVS convertible to 1,276,208 outstanding for a total outstanding SVS of 593,904,174 on an as-converted basis. Please see Section 10 - Description of Securities of 4Front's Form 2A Listing Statement for a further description of the SVS and MVS. Each Warrant entitles the holder thereof, upon exercise 4Front Ventures at any time after the Issue Date and prior to the Expiry US Corp. -

CANNABIS STRAIN LIST Lab Tested by NUTRACEUTICAL SCIENCE LABORATORIES
CANNABIS STRAIN LIST Lab tested by NUTRACEUTICAL SCIENCE LABORATORIES SUPER LEMON HAZE TANGILOPE BIG BUD LEMON SKUNK x SUPER SILVER HAZE CHOCOLOPE x TANGIE AFGHANI x NORTHERN LIGHTS x SKUNK #1 Green House Seeds DNA Genetics Sensi Seeds The effects of Super Lemon Haze This sativa cultivar is extremely This indica dominant strain has are extremely euphoric and heavy on the Tangie side of the a pungent citrus taste with a energetic, providing a very alert terpene spectrum. Providing a clean pine after taste. buzz. It is an idyllic daytime bouquet of fresh squeezed The genetics of this anxiety medicine. The taste is orange juice while maintaining relieving strain include Afghani, extraordinarily sweet and citrusy the structure and yield of the Skunk#1, and Northern Lights. with a distinct lemon flavor. Chocolope lineage. Known for assisting with Known for assisting with Known for assisting with severe pain, depression, PTSD stress, depression, depression, fatigue, stress, fatigue, pain, digestion, pain, lack of appetite muscle spasm sativa sativa indica THC 15.75 - 21.01% THC 10 - 22% THC 13 - 22.24% SOUR DIESEL (AJ’s Cut) GRADY’S KUSH WEDDING CAKE BX1 LINEAGE UNKNOWN GRADY’S SPECIAL PURPLE TRAINWRECK TRIANGLE MINTS (WEDDING CAKE CUT) x TRAINWRECK x TRIANGLE MINTS SeedJunky Genetics Sour Diesel gets its name from A pungent strain infused with the pungent mix of pine, lemon pine and citrus aromas. Creates Our Wedding Cake leans very and diesel fuel smell it gives off. a strong cerebral focus and heavily to the earthy Kush side of A sativa dominant hybrid, uplifting mental clarity, while its lineage.