Survival and Development of Lymantria Monacha (Lepidoptera: Lymantriidae) on North American and Introduced Eurasian Tree Species
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Coastal Landscaping in Massachusetts Plant List
Coastal Landscaping in Massachusetts Plant List This PDF document provides additional information to supplement the Massachusetts Office of Coastal Zone Management (CZM) Coastal Landscaping website. The plants listed below are good choices for the rugged coastal conditions of Massachusetts. The Coastal Beach Plant List, Coastal Dune Plant List, and Coastal Bank Plant List give recommended species for each specified location (some species overlap because they thrive in various conditions). Photos and descriptions of selected species can be found on the following pages: • Grasses and Perennials • Shrubs and Groundcovers • Trees CZM recommends using native plants wherever possible. The vast majority of the plants listed below are native (which, for purposes of this fact sheet, means they occur naturally in eastern Massachusetts). Certain non-native species with specific coastal landscaping advantages that are not known to be invasive have also been listed. These plants are labeled “not native,” and their state or country of origin is provided. (See definitions for native plant species and non-native plant species at the end of this fact sheet.) Coastal Beach Plant List Plant List for Sheltered Intertidal Areas Sheltered intertidal areas (between the low-tide and high-tide line) of beach, marsh, and even rocky environments are home to particular plant species that can tolerate extreme fluctuations in water, salinity, and temperature. The following plants are appropriate for these conditions along the Massachusetts coast. Black Grass (Juncus gerardii) native Marsh Elder (Iva frutescens) native Saltmarsh Cordgrass (Spartina alterniflora) native Saltmeadow Cordgrass (Spartina patens) native Sea Lavender (Limonium carolinianum or nashii) native Spike Grass (Distichlis spicata) native Switchgrass (Panicum virgatum) native Plant List for a Dry Beach Dry beach areas are home to plants that can tolerate wind, wind-blown sand, salt spray, and regular interaction with waves and flood waters. -

Gypsy Moth CP
INDUSTRY BIOSECURITY PLAN FOR THE NURSERY & GARDEN INDUSTRY Threat Specific Contingency Plan Gypsy moth (Asian and European strains) Lymantria dispar dispar Plant Health Australia December 2009 Disclaimer The scientific and technical content of this document is current to the date published and all efforts were made to obtain relevant and published information on the pest. New information will be included as it becomes available, or when the document is reviewed. The material contained in this publication is produced for general information only. It is not intended as professional advice on any particular matter. No person should act or fail to act on the basis of any material contained in this publication without first obtaining specific, independent professional advice. Plant Health Australia and all persons acting for Plant Health Australia in preparing this publication, expressly disclaim all and any liability to any persons in respect of anything done by any such person in reliance, whether in whole or in part, on this publication. The views expressed in this publication are not necessarily those of Plant Health Australia. Further information For further information regarding this contingency plan, contact Plant Health Australia through the details below. Address: Suite 5, FECCA House 4 Phipps Close DEAKIN ACT 2600 Phone: +61 2 6215 7700 Fax: +61 2 6260 4321 Email: [email protected] Website: www.planthealthaustralia.com.au PHA & NGIA | Contingency Plan – Asian and European gypsy moth (Lymantria dispar dispar) 1 Purpose and background of this contingency plan .............................................................. 5 2 Australian nursery industry .................................................................................................... 5 3 Eradication or containment determination ............................................................................ 6 4 Pest information/status .......................................................................................................... -
Birch Defoliator Yukon Forest Health — Forest Insect and Disease 4
Birch Defoliator Yukon Forest Health — Forest insect and disease 4 Energy, Mines and Resources Forest Management Branch Introduction The birch leafminer (Fenusa pusilla), amber-marked birch leafminer (Profenusa thomsoni), birch leaf skeletonizer (Bucculatrix canadensisella) and the birch-aspen leafroller (Epinotia solandriana) are defoliators of white birch (Betula papyrifera) in North America. Of the four, only the Bucculatrix is native to North America, but it is not currently found in Yukon. The other three species, as invasives, pose a far greater threat to native trees because their natural enemies in the form of predators, parasites and diseases are absent here. The birch leafminer was accidently introduced from Europe in 1923 and is now widely distributed in Canada, Alaska and the northern United States, though it has not yet been found in Yukon. The amber-marked birch leafminer was first described in Quebec in 1959 but is now found throughout Canada, the northern contiguous U.S., and Alaska. The amber-marked birch leafminer has proven to be, by far, the more damaging of the two species. Both species are of the blotch mining type as opposed to the skeletonizing Bucculatrix and the leafrolling Epinotia. Amber-marked leafminer damage is typically found along road systems. Infestations along roadsides are often greater in areas of high traffic, or where parked cars are common, suggesting that this pest will hitchhike on vehicles. It was first identified in Anchorage, Alaska in 1996 and has since spread widely to other communities. In areas of Alaska, efforts to control the spread of the amber-marked birch leafminer have been underway since 2003 with the release of parasitic wasps (Lathrolestes spp.). -

Strategies for the Eradication Or Control of Gypsy Moth in New Zealand
Strategies for the eradication or control of gypsy moth in New Zealand Travis R. Glare1, Patrick J. Walsh2*, Malcolm Kay3 and Nigel D. Barlow1 1 AgResearch, PO Box 60, Lincoln, New Zealand 2 Forest Research Associates, Rotorua (*current address Galway-Mayo Institute of Technology, Dublin Road, Galway, Republic of Ireland) 3Forest Research, Private Bag 3020, Rotorua Efforts to remove gypsy moth from an elm, Malden, MA, circa 1891 May 2003 STATEMENT OF PURPOSE The aim of the report is to provide background information that can contribute to developing strategies for control of gypsy moth. This is not a contingency plan, but a document summarising the data collected over a two year FRST-funded programme on biological control options for gypsy moth relevant to New Zealand, completed in 1998 and subsequent research on palatability of New Zealand flora to gypsy moth. It is mainly aimed at discussing control options. It should assist with rapidly developing a contingency plan for gypsy moth in the case of pest incursion. Abbreviations GM gypsy moth AGM Asian gypsy moth NAGM North America gypsy moth EGM European gypsy moth Bt Bacillus thuringiensis Btk Bacillus thuringiensis kurstaki MAF New Zealand Ministry of Agriculture and Forestry MOF New Zealand Ministry of Forestry (defunct, now part of MAF) NPV nucleopolyhedrovirus LdNPV Lymantria dispar nucleopolyhedrovirus NZ New Zealand PAM Painted apple moth, Teia anartoides FR Forest Research PIB Polyhedral inclusion bodies Strategies for Asian gypsy moth eradication or control in New Zealand page 2 SUMMARY Gypsy moth, Lymantria dispar (Lepidoptera: Lymantriidae), poses a major threat to New Zealand forests. It is known to attack over 500 plant species and has caused massive damage to forests in many countries in the northern hemisphere. -

The Tree How to Identify a Linden (Tilia Spp.) the Pesticides the Pest
The Tree Tilia cordata, the Littleleaf Linden tree is native to Europe. It has been at the center of several bumble bee kills in Oregon. T. cordata often produces more flowers than other linden trees. It also produces mannose in its nectar that may be slightly toxic. Many native bees and wasps do not have the enzyme to break down mannose. European honey bees, Apis mellifera, do not appear to be as affected by mannose; at least one theory is that because they are from Europe, they share a developmental history with T. cordata. In general, linden trees have few pest problems; aphids are listed as one of the only insect pests of Tilia trees. Tilia leaf comparison How to Identify a Linden (Tilia spp.) DURING THE WINTER/DORMANT SEASON: 1. Bark is gray-brown and on mature trees is ridged or plated. 2. Twigs are light brown to gray, or may be red-tinged. 3. Buds are prominent, single, plump and often bulge on one side, and are red-brown to dark red in color. 4. Floral bracts and fruit may remain on the tree through winter. DURING THE GROWING SEASON: 1. Leaves are singular, alternate, heart-shaped, finely toothed, and the undersides of leaves often are fuzzy. Leaves at the stem end are asymmetrically attached to the stem. 2. Flowers are attached by floral bract that is 2-to-4 inches long. White to yellow flowers with five petals in hanging clusters of five-to-seven bloom in mid-June or early July. Flowers are fragrant and highly attractive to pollinators. -

Littleleaf Linden—Loved by Bees
Littleleaf Linden—Loved by Bees By Susan Camp In last week’s “Gardening Corner,” I wrote about a weeping Higan cherry (Prunus subhirtella ‘Pendula”) that is struggling, most likely because it is too closely located to several other trees that block its access to sunlight. Two of the guilty trees are littleleaf lindens (Tilia cordata), members of the Malvaceae or mallow family and native to Europe and southwestern Asia. Littleleaf lindens also are called small-leaved lindens. In Britain, they are known as lime trees, although they aren’t related to the citrus tree and fruit that bear the same name. Several other species of linden exist. Three littleleaf lindens were planted on our property by the previous owners more than 30 years ago. They have a good chance to live several hundred years if they escape severe disease, insect infestation, or environmental changes. In fact, longevity may be one of the reasons lindens were planted along streets and avenues in European, and later, American cities. Lindens also make reliable city trees because they tolerate poor or compacted soil and air pollution. In addition, the trees withstand occasional drought conditions, although leaf margins may scorch in prolonged heat. Newly planted trees should be watered regularly during the first years. Littleleaf lindens grow in USDA Hardiness Zones 3 to 7 and don’t perform as well in warmer zones. The trees prefer full sun to part shade in average sandy soil or loam with a pH of 4.5 to 8.2, which means they will tolerate acidic to mildly alkaline soil. -

Tilia Cordata 'Greenspire'
Fact Sheet ST-639 October 1994 Tilia cordata ‘Greenspire’ ‘Greenspire’ Littleleaf Linden1 Edward F. Gilman and Dennis G. Watson2 INTRODUCTION ‘Greenspire’ Littleleaf Linden grows 50 to 75 feet tall and can spread 40 to 50 feet, but is normally seen 40 to 50 feet tall with a 35 to 40-foot-spread in most landscapes (Fig. 1). This tree has a faster growth rate than the species and a dense pyramidal to oval crown which casts deep shade. The leaves are smaller than the species adding a delicate touch to the tree. From a distance the tree almost resembles a narrow version of the Bradford Callery Pear. This cultivar of Littleleaf Linden is more popular than the species or any of the other cultivars. It is a prolific bloomer, the small fragrant flowers appearing in late June and into July. Many bees are attracted to the flowers, and the dried flowers persist on the tree for some time. Japanese beetles often skeletonize Linden foliage, in certain areas in the northern part of its range. Defoliation can be nearly total and mature trees can be killed by severe infestations. Planting Linden in areas with severe infestations of this pest may not be wise. However, at least one reference reports that defoliation by Japanese beetles is common but control is seldom needed. GENERAL INFORMATION Scientific name: Tilia cordata ‘Greenspire’ Pronunciation: TILL-ee-uh kor-DAY-tuh Figure 1. Middle-aged ‘Greenspire’ Littleleaf Linden. Common name(s): ‘Greenspire’ Littleleaf Linden Family: Tiliaceae tree lawns (>6 feet wide); medium-sized tree lawns USDA hardiness zones: 3 through 7A (Fig. -

The HERITAGE River Birch (Betula Nigra 'Cully') a Notable Landscape
1 Collyer Lane, Basking Ridge, NJ 07920 email: [email protected] Website: http://www.bernards.org/boards_commissions/shade_tree/default.aspx The HERITAGE River Birch (Betula nigra 'Cully') A notable landscape tree is the HERITAGE river birch (Betula nigra 'Cully'). This tree has gained a great deal of popularity and has been planted extensively in residential settings over the last ten years. It can be identified by the peeling tan bark of various shades. Most examples are multi-trunked in clumps of three to five. It is a native tree that grows both in the southern and northern areas of the United States. The cultivar HERITAGE was found by Earl Cully, a nurseryman from Illinois, who is well recognized for development of various worthwhile landscape trees. He found a birch tree that had exceptional peeling bark flourishing on a residential lawn in a St. Louis suburb more than two decades ago. River birch grow in moist, but not constantly wet areas, along streams and rivers. Other native birches in this area are the yellow birch which has curly black to gray peeling bark and the gray birch, Betula populifolia, which has chalky white non- peeling bark. In colder areas of the Northeast one may note white birch that in time develops peeling bark; these are non-native. The European white weeping birch, Betula pendula, is also useful in the landscape; it attains a good height and is strongly weeping. This is common plant to see European landscapes. Birch trees are also native to Asia and some have outstanding white bark and make wonderful specimen trees in colder areas of the Northeast. -

(Betula Populifolia) R
PRODUCTION AND PROPERTIES OF BIRCH SYRUP (Betula populifolia) R. Kok1, E.R. Norris1, and T. Beveridge2 1Department of Agricultural Engineering, and 2SchoolofFood Science. Macdonald College of McGillUniversity, Ste. Annede Bellevue, Quebec HOA ICO Received 30 November 1976 Kok, R., E.R. Norris, and T. Beveridge. 1978. Production and properties of birchsyrup(Betulapopulifolia). Can. Agric. Eng. 20: 5-9. Sixteen grey birch trees ( B. populifolia) were tapped during 1975; fifty were tapped during 1976. Sapwas collected daily and boiled to a syrup. Thesugar content as well as the volume of thesapproduced byeachtreewas measured every dayduring the 1976 season. Theaverage seasonal sap production during 1975 was 42.8 //tree;during 1976 it was 27.9 //tree.Theaverage sap sugarcontent during 1976 was 0.74% (w/v). Trees tapped suffered noapparentilleffects from thetapping. Average syrup/;Hwas 4.8. The average syrup (50% byweight sugar) viscosity was 15 centipoise. Thesyrup ash content increased during the tapping season from 1.22 to 3.3%. Thedominant wavelength ofthesyrup color was 580 nm; itsexcitation purity was 0.671. The syrup was judged as being acceptable by 59%, of an 82-member taste panel. INTRODUCTION birch), B. papyri/era (white birch) and B. combined and kept for boiling. alleghaniensis (yellow birch) (Marie- The solids content of the daily individual Although maple products and their Victorin 1964; Hosie 1969). For this study, characteristic flavor are today well known, tree sap samples was determined by the grey birch was chosen, since it was similar products obtainable from birch, pipetting 10 ml of sap into a drying dish, abundantly available in the immediate walnut, hickory, ash, basswood and leaving the dish in a drying oven at 75°C for vicinity. -
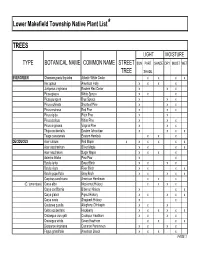
Native Plant List Trees.XLS
Lower Makefield Township Native Plant List* TREES LIGHT MOISTURE TYPE BOTANICAL NAME COMMON NAME STREET SUN PART SHADE DRY MOIST WET TREE SHADE EVERGREEN Chamaecyparis thyoides Atlantic White Cedar x x x x IIex opaca American Holly x x x x Juniperus virginiana Eastern Red Cedar x x x Picea glauca White Spruce x x x Picea pungens Blue Spruce x x x Pinus echinata Shortleaf Pine x x x Pinus resinosa Red Pine x x x Pinus rigida Pitch Pine x x Pinus strobus White Pine x x x Pinus virginiana Virginia Pine x x x Thuja occidentalis Eastern Arborvitae x x x x Tsuga canadensis Eastern Hemlock xx x DECIDUOUS Acer rubrum Red Maple x x x x x x Acer saccharinum Silver Maple x x x x Acer saccharum Sugar Maple x x x x Asimina triloba Paw-Paw x x Betula lenta Sweet Birch x x x x Betula nigra River Birch x x x x Betula populifolia Gray Birch x x x x x Carpinus caroliniana American Hornbeam x x x (C. tomentosa) Carya alba Mockernut Hickory x x x x Carya cordiformis Bitternut Hickory x x x Carya glabra Pignut Hickory x x x x x Carya ovata Shagbark Hickory x x Castanea pumila Allegheny Chinkapin xx x Celtis occidentalis Hackberry x x x x x x Crataegus crus-galli Cockspur Hawthorn x x x x Crataegus viridis Green Hawthorn x x x x Diospyros virginiana Common Persimmon x x x x Fagus grandifolia American Beech x x x x PAGE 1 Exhibit 1 TREES (cont'd) LIGHT MOISTURE TYPE BOTANICAL NAME COMMON NAME STREET SUN PART SHADE DRY MOIST WET TREE SHADE DECIDUOUS (cont'd) Fraxinus americana White Ash x x x x Fraxinus pennsylvanica Green Ash x x x x x Gleditsia triacanthos v. -

Die Winterlinde (Tilia Cordata): Verwandtschaft, Morphologie Und Ökologie Gregor Aas
Die Winterlinde (Tilia cordata): Verwandtschaft, Morphologie und Ökologie Gregor Aas Schlüsselwörter: Tilia cordata, Taxonomie, Morphologie, Beide Linden sind als Waldbäume bei uns weit verbrei- Ökologie, Blütenbiologie tet, kommen aber immer nur vereinzelt oder in kleinen Gruppen vor. Selten treten sie bestandsbildend auf grö- Zusammenfassung: Die Winterlinde (Tilia cordata, Malva- ßerer Fläche auf. Häufig sind sie außerhalb des Waldes ceae, Malvengewächse, Unterfamilie Tilioideae, Linden- gepflanzt, beispielsweise als Dorflinden, als Solitäre an gewächse) ist neben der Sommerlinde (T. platyphyllos) die Kirchen und Kapellen oder in Alleen (Abbildungen 1 zweite in Mitteleuropa einheimische Lindenart. Darge- und 2). Viele Sagen, Mythen, Gebräuche und Orts- stellt werden neben der Verbreitung, der Morphologie, namen, die auf die Linde zurückgehen, belegen ihre der Ökologie und der Reproduktionsbiologie der Winter- große kulturelle Bedeutung im Leben der Menschen linde, insbesondere die Unterscheidung von der Sommer- früherer Jahrhunderte. Diese Wertschätzung beruhte linde. auch auf den vielfältigen Nutzungen. Das Holz war be- gehrt in der Schnitzerei, der Bast als Bindematerial lan- Die Gattung Tilia und die bei uns vorkom- menden Arten Zu den Linden (Tilia, Familie Malvengewächse, Mal- vaceae, Unterfamilie Lindengewächse, Tilioideae) gehören etwa 25 sommergrüne Baum- und Strauchar- ten, die in der gemäßigten Zone der Nordhemisphäre verbreitet sind. In Mitteleuropa sind zwei Arten einhei- misch, die Winterlinde (Tilia cordata MILL.) und die Sommerlinde (T. platyphyllos SCOP.). Abbildung 1 (oben): Winterlinde am so genannten »Käppele« bei Dettighofen nahe der schweizer Grenze im südbadischen Klettgau Foto: G. Aas Abbildung 2 (links): Allee mit Winter- und Sommerlinden am Weg zur Burg Wiesentfels im Tal der Wiesent (nördliche Frankenalb) Foto: H. Steinecke LWF Wissen 78 7 Die Winterlinde (Tilia cordata): Verwandtschaft, Morphologie und Ökologie ge Zeit unersetzlich und die Blätter und Blüten wurden für Heilzwecke verwendet. -

Fatty Acid Composition of Tilia Spp. Seed Oils
GRASAS Y ACEITES, 64 (3), ABRIL-JUNIO, 243-249, 2013, ISSN: 0017-3495 DOI: 10.3989/gya.096012 Fatty acid composition of Tilia spp. seed oils By M.K. Dowda, * and M.C. Farvea a Southern Regional Research Center, Agricultural Research Service, U.S. Department of Agriculture, 1100 Robert E. Lee Blvd. New Orleans, LA, 70124 USA * Corresponding author: [email protected] RESUMEN Two additional a-oxidation products, 8-heptadecenoic acid and 8,11-heptadecadienoic acid were also detected. Composición en ácidos grasos de aceites de semi- Combined, the level of these fatty acids was between 1.3 llas de Tilia spp. and 2.3%, roughly comparable to the levels of these acids recently reported in the seed oil of Thespesia populnea. Como parte de un estudio sobre la composición de aceites derivados de semillas de plantas Malvaceae, las semillas de KEY-WORDS: a-Oxidized fatty acids – Cyclopropenoid siete especies de Tilia (árboles de tilia o lima) fueron evalua- fatty acids – Lime trees – Linden trees. das con respecto a sus perfiles de ácidos grasos. Las semillas fueron obtenidas de Germplasm Research Information Net- work así como de varias fuentes comerciales. Tras la extrac- ción del aceite con hexano, los glicéridos fueron trans-metila- 1. INTRODUCTION dos y analizados por cromatografía de gases con dos fases polares estacionarias. Todos los aceites extraidos de las semi- As part of a broad study of the seed oil fatty acid llas analizados estaban compuestos principalmente de ácido composition of Malvaceae plants, several species linoleico (49-60%) y, en cantidades más bajas de ácido oleico within the Tilia genus were evaluated.