A Case of Achondrogenesis Type 2/ Hypochondrogenesis Due to Ade
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Orphanet Report Series Rare Diseases Collection
Marche des Maladies Rares – Alliance Maladies Rares Orphanet Report Series Rare Diseases collection DecemberOctober 2013 2009 List of rare diseases and synonyms Listed in alphabetical order www.orpha.net 20102206 Rare diseases listed in alphabetical order ORPHA ORPHA ORPHA Disease name Disease name Disease name Number Number Number 289157 1-alpha-hydroxylase deficiency 309127 3-hydroxyacyl-CoA dehydrogenase 228384 5q14.3 microdeletion syndrome deficiency 293948 1p21.3 microdeletion syndrome 314655 5q31.3 microdeletion syndrome 939 3-hydroxyisobutyric aciduria 1606 1p36 deletion syndrome 228415 5q35 microduplication syndrome 2616 3M syndrome 250989 1q21.1 microdeletion syndrome 96125 6p subtelomeric deletion syndrome 2616 3-M syndrome 250994 1q21.1 microduplication syndrome 251046 6p22 microdeletion syndrome 293843 3MC syndrome 250999 1q41q42 microdeletion syndrome 96125 6p25 microdeletion syndrome 6 3-methylcrotonylglycinuria 250999 1q41-q42 microdeletion syndrome 99135 6-phosphogluconate dehydrogenase 67046 3-methylglutaconic aciduria type 1 deficiency 238769 1q44 microdeletion syndrome 111 3-methylglutaconic aciduria type 2 13 6-pyruvoyl-tetrahydropterin synthase 976 2,8 dihydroxyadenine urolithiasis deficiency 67047 3-methylglutaconic aciduria type 3 869 2A syndrome 75857 6q terminal deletion 67048 3-methylglutaconic aciduria type 4 79154 2-aminoadipic 2-oxoadipic aciduria 171829 6q16 deletion syndrome 66634 3-methylglutaconic aciduria type 5 19 2-hydroxyglutaric acidemia 251056 6q25 microdeletion syndrome 352328 3-methylglutaconic -

Blueprint Genetics Comprehensive Growth Disorders / Skeletal
Comprehensive Growth Disorders / Skeletal Dysplasias and Disorders Panel Test code: MA4301 Is a 374 gene panel that includes assessment of non-coding variants. This panel covers the majority of the genes listed in the Nosology 2015 (PMID: 26394607) and all genes in our Malformation category that cause growth retardation, short stature or skeletal dysplasia and is therefore a powerful diagnostic tool. It is ideal for patients suspected to have a syndromic or an isolated growth disorder or a skeletal dysplasia. About Comprehensive Growth Disorders / Skeletal Dysplasias and Disorders This panel covers a broad spectrum of diseases associated with growth retardation, short stature or skeletal dysplasia. Many of these conditions have overlapping features which can make clinical diagnosis a challenge. Genetic diagnostics is therefore the most efficient way to subtype the diseases and enable individualized treatment and management decisions. Moreover, detection of causative mutations establishes the mode of inheritance in the family which is essential for informed genetic counseling. For additional information regarding the conditions tested on this panel, please refer to the National Organization for Rare Disorders and / or GeneReviews. Availability 4 weeks Gene Set Description Genes in the Comprehensive Growth Disorders / Skeletal Dysplasias and Disorders Panel and their clinical significance Gene Associated phenotypes Inheritance ClinVar HGMD ACAN# Spondyloepimetaphyseal dysplasia, aggrecan type, AD/AR 20 56 Spondyloepiphyseal dysplasia, Kimberley -

Blueprint Genetics Comprehensive Skeletal Dysplasias and Disorders
Comprehensive Skeletal Dysplasias and Disorders Panel Test code: MA3301 Is a 251 gene panel that includes assessment of non-coding variants. Is ideal for patients with a clinical suspicion of disorders involving the skeletal system. About Comprehensive Skeletal Dysplasias and Disorders This panel covers a broad spectrum of skeletal disorders including common and rare skeletal dysplasias (eg. achondroplasia, COL2A1 related dysplasias, diastrophic dysplasia, various types of spondylo-metaphyseal dysplasias), various ciliopathies with skeletal involvement (eg. short rib-polydactylies, asphyxiating thoracic dysplasia dysplasias and Ellis-van Creveld syndrome), various subtypes of osteogenesis imperfecta, campomelic dysplasia, slender bone dysplasias, dysplasias with multiple joint dislocations, chondrodysplasia punctata group of disorders, neonatal osteosclerotic dysplasias, osteopetrosis and related disorders, abnormal mineralization group of disorders (eg hypopohosphatasia), osteolysis group of disorders, disorders with disorganized development of skeletal components, overgrowth syndromes with skeletal involvement, craniosynostosis syndromes, dysostoses with predominant craniofacial involvement, dysostoses with predominant vertebral involvement, patellar dysostoses, brachydactylies, some disorders with limb hypoplasia-reduction defects, ectrodactyly with and without other manifestations, polydactyly-syndactyly-triphalangism group of disorders, and disorders with defects in joint formation and synostoses. Availability 4 weeks Gene Set Description -

REVIEW ARTICLE Genetic Disorders of the Skeleton: a Developmental Approach
Am. J. Hum. Genet. 73:447–474, 2003 REVIEW ARTICLE Genetic Disorders of the Skeleton: A Developmental Approach Uwe Kornak and Stefan Mundlos Institute for Medical Genetics, Charite´ University Hospital, Campus Virchow, Berlin Although disorders of the skeleton are individually rare, they are of clinical relevance because of their overall frequency. Many attempts have been made in the past to identify disease groups in order to facilitate diagnosis and to draw conclusions about possible underlying pathomechanisms. Traditionally, skeletal disorders have been subdivided into dysostoses, defined as malformations of individual bones or groups of bones, and osteochondro- dysplasias, defined as developmental disorders of chondro-osseous tissue. In light of the recent advances in molecular genetics, however, many phenotypically similar skeletal diseases comprising the classical categories turned out not to be based on defects in common genes or physiological pathways. In this article, we present a classification based on a combination of molecular pathology and embryology, taking into account the importance of development for the understanding of bone diseases. Introduction grouping of conditions that have a common molecular origin but that have little in common clinically. For ex- Genetic disorders affecting the skeleton comprise a large ample, mutations in COL2A1 can result in such diverse group of clinically distinct and genetically heterogeneous conditions as lethal achondrogenesis type II and Stickler conditions. Clinical manifestations range from neonatal dysplasia, which is characterized by moderate growth lethality to only mild growth retardation. Although they retardation, arthropathy, and eye disease. It is now be- are individually rare, disorders of the skeleton are of coming increasingly clear that several distinct classifi- clinical relevance because of their overall frequency. -

Syndrome of the Month J Med Genet: First Published As 10.1136/Jmg.33.11.957 on 1 November 1996
J Med Genet 1996;33:957-961 957 Syndrome of the month J Med Genet: first published as 10.1136/jmg.33.11.957 on 1 November 1996. Downloaded from Achondrogenesis type 1B Andrea Superti-Furga Historical notes which tried to provide a quantitative basis (the In 1952, the name achondrogenesis (Greek for "femoral cylinder index") for qualitative "not producing cartilage") was given by Marco changes, did not prove helpful and was later Fraccaro, a young Italian pathologist (later to abandoned. In the late 1980s, it was shown become a well known cytogeneticist), to the that achondrogenesis type II was caused by condition he observed in a stillborn female structural mutations in collagen II and thus with severe micromelia and marked histological constituted the severe end of the spectrum of changes of cartilage.' Fraccaro noted a similar the collagen II chondrodysplasias.l'" case published by Parenti in 1936.2 Fraccaro's Borochowitz et al'4 provided convincing report (written in Italian) came to the know- histological criteria for the further subdivision ledge ofHans Grebe, who in 1939 had observed of achondrogenesis type I into IA (with ap- sisters (aged 7 and 11 years, born to con- parently normal cartilage matrix but inclusions sanguineous parents from the Black Forest re- in chondrocytes) and IB (with abnormal car- gion of Germany) with markedly short limbs tilage matrix; see below). These findings were and digits but normal trunk. Grebe became confirmed by another group shortly there- convinced that his patients were affected by after.'5 Using -
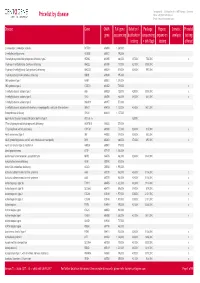
Pricelist by Disease Phone: +49 (0)381 203 652 222 E-Mail: [email protected]
Centogene AG :: Schillingallee 68 :: 18057 Rostock :: Germany Pricelist by disease Phone: +49 (0)381 203 652 222 E-mail: [email protected] Disease Gene OMIM Full gene Deletion/ Package Repeat Somatic Prenatal gene sequencing duplication (sequencing expansion analysis testing testing + del/dup) testing offered 2-aminoadipic 2-oxoadipic aciduria DHTKD1 614984 1.260,00 € 2-methylbutyrylglycinuria ACADSB 600301 796,00 € 3-beta-hydroxysteroid dehydrogenase deficiency type 2 HSD3B2 613890 468,00 € 437,00 € 755,00 € x 3-hydroxy-3-methylglutaryl-CoA lyase deficiency HMGCL 613898 737,00 € 421,00 € 1.008,00 € 3-hydroxy-3-methylglutaryl-CoA synthase 2 deficiency HMGCS2 600234 819,00 € 328,00 € 997,00 € 3-hydroxyisobutryl-CoA hydrolase deficiency HIBCH 610690 995,00 € 3MC syndrome type 1 MASP1 600521 1.278,00 € 3MC syndrome type 2 COLEC11 612502 729,00 € x 3-methylglutaconic aciduria type 1 AUH 600529 729,00 € 429,00 € 1.008,00 € x 3-methylglutaconic aciduria type 3 OPA3 606580 468,00 € 343,00 € 661,00 € x 3-methylglutaconic aciduria type 5 DNAJC19 608977 573,00 € 3-methylglutaconic aciduria with deafness, encephalopathy, and Leigh-like syndrome SERAC1 614725 1.127,00 € 424,00 € 1.401,00 € 5-oxoprolinase deficiency OPLAH 614243 1.127,00 € 6q24-related transient neonatal diabetes mellitus type 1 UPD chr. 6 328,00 € 17-beta hydroxysteroid dehydrogenase X deficiency HSD17B10 300256 573,00 € 17-hydroxylation activity deficiency CYP17A1 609300 737,00 € 328,00 € 915,00 € x 46,XX sex reversal type 1 SRY 480000 374,00 € 328,00 € 552,00 € 46,XY gonadal -

Skeletal Dysplasias Precision Panel Overview Indications
Skeletal Dysplasias Precision Panel Overview Skeletal Dysplasias, also known as osteochondrodysplasias, are a clinically and phenotypically heterogeneous group of more than 450 inherited disorders characterized by abnormalities mainly of cartilage and bone growth although they can also affect muscle, tendons and ligaments, resulting in abnormal shape and size of the skeleton and disproportion of long bones, spine and head. They differ in natural histories, prognoses, inheritance patterns and physiopathologic mechanisms. They range in severity from those that are embryonically lethal to those with minimum morbidity. Approximately 5% of children with congenital birth defects have skeletal dysplasias. Until recently, the diagnosis of skeletal dysplasia relied almost exclusively on careful phenotyping, however, the advent of genomic tests has the potential to make a more accurate and definite diagnosis based on the suspected clinical diagnosis. The 4 most common skeletal dysplasias are thanatophoric dysplasia, achondroplasia, osteogenesis imperfecta and achondrogenesis. The inheritance pattern of skeletal dysplasias is variable and includes autosomal dominant, recessive and X-linked. The Igenomix Skeletal Dysplasias Precision Panel can be used to make a directed and accurate differential diagnosis of skeletal abnormalities ultimately leading to a better management and prognosis of the disease. It provides a comprehensive analysis of the genes involved in this disease using next-generation sequencing (NGS) to fully understand the spectrum -

Whole Exome Sequencing Gene Package Skeletal Dysplasia, Version 2.1, 31-1-2020
Whole Exome Sequencing Gene package Skeletal Dysplasia, Version 2.1, 31-1-2020 Technical information DNA was enriched using Agilent SureSelect DNA + SureSelect OneSeq 300kb CNV Backbone + Human All Exon V7 capture and paired-end sequenced on the Illumina platform (outsourced). The aim is to obtain 10 Giga base pairs per exome with a mapped fraction of 0.99. The average coverage of the exome is ~50x. Duplicate and non-unique reads are excluded. Data are demultiplexed with bcl2fastq Conversion Software from Illumina. Reads are mapped to the genome using the BWA-MEM algorithm (reference: http://bio-bwa.sourceforge.net/). Variant detection is performed by the Genome Analysis Toolkit HaplotypeCaller (reference: http://www.broadinstitute.org/gatk/). The detected variants are filtered and annotated with Cartagenia software and classified with Alamut Visual. It is not excluded that pathogenic mutations are being missed using this technology. At this moment, there is not enough information about the sensitivity of this technique with respect to the detection of deletions and duplications of more than 5 nucleotides and of somatic mosaic mutations (all types of sequence changes). HGNC approved Phenotype description including OMIM phenotype ID(s) OMIM median depth % covered % covered % covered gene symbol gene ID >10x >20x >30x ABCC9 Atrial fibrillation, familial, 12, 614050 601439 65 100 100 95 Cardiomyopathy, dilated, 1O, 608569 Hypertrichotic osteochondrodysplasia, 239850 ACAN Short stature and advanced bone age, with or without early-onset osteoarthritis -

EUROCAT Syndrome Guide
JRC - Central Registry european surveillance of congenital anomalies EUROCAT Syndrome Guide Definition and Coding of Syndromes Version July 2017 Revised in 2016 by Ingeborg Barisic, approved by the Coding & Classification Committee in 2017: Ester Garne, Diana Wellesley, David Tucker, Jorieke Bergman and Ingeborg Barisic Revised 2008 by Ingeborg Barisic, Helen Dolk and Ester Garne and discussed and approved by the Coding & Classification Committee 2008: Elisa Calzolari, Diana Wellesley, David Tucker, Ingeborg Barisic, Ester Garne The list of syndromes contained in the previous EUROCAT “Guide to the Coding of Eponyms and Syndromes” (Josephine Weatherall, 1979) was revised by Ingeborg Barisic, Helen Dolk, Ester Garne, Claude Stoll and Diana Wellesley at a meeting in London in November 2003. Approved by the members EUROCAT Coding & Classification Committee 2004: Ingeborg Barisic, Elisa Calzolari, Ester Garne, Annukka Ritvanen, Claude Stoll, Diana Wellesley 1 TABLE OF CONTENTS Introduction and Definitions 6 Coding Notes and Explanation of Guide 10 List of conditions to be coded in the syndrome field 13 List of conditions which should not be coded as syndromes 14 Syndromes – monogenic or unknown etiology Aarskog syndrome 18 Acrocephalopolysyndactyly (all types) 19 Alagille syndrome 20 Alport syndrome 21 Angelman syndrome 22 Aniridia-Wilms tumor syndrome, WAGR 23 Apert syndrome 24 Bardet-Biedl syndrome 25 Beckwith-Wiedemann syndrome (EMG syndrome) 26 Blepharophimosis-ptosis syndrome 28 Branchiootorenal syndrome (Melnick-Fraser syndrome) 29 CHARGE -

Mouse Models of Human Disease. Part II: Recent Progress and Future Directions
Downloaded from genesdev.cshlp.org on September 30, 2021 - Published by Cold Spring Harbor Laboratory Press Mouse models of human disease. Part II: Recent progress and future directions Mary A. Bedell, 1 David A. Largaespada, 2 Nancy A. Jenkins, and Neal G. Copeland 3 Mammalian Genetics Laboratory, ABL-Basic Research Program, NCI-Frederick Cancer Research and Development Center, Frederick, Maryland 21702-1201 USA The development of new methods for manipulating the the recent progress in this area. Throughout the text and mouse genome, including transgenic and embryonic tables, we have cited only the most recent papers and stem (ES) cell knockout technology, combined with refer to reviews whenever possible. Interested readers are greatly improved genetic and physical maps for mouse encouraged to read the primary papers on each model has revolutionized our ability to generate new mouse and associated disease. models of human disease. In Part I of this review (Bedell et al., this issue), we described in detail the various tech- Disorders of neural crest derivatives niques and genetic resources that have facilitated mouse model development. In Part II of this review we highlight Cells from the neural crest differentiate into many dif- some of the recent progress that has been made in mouse ferent cell types including melanocytes of the skin and model development and discuss areas where these inner ear, neuronal and glial components of the periph- mouse models are likely to contribute in the future. We eral nervous system, neuroendocrine cells of the adrenal have focused in part II only on those models where the medulla and thyroid, and cartilaginous and membranous homologous gene is mutated in both the human and bones of the skull. -

COL2A1 Mutations with Analysis of Genotype-Phenotype J Med Genet: First Published As 10.1136/Jmg.37.4.263 on 1 April 2000
J Med Genet 2000;37:263–271 263 Report of five novel and one recurrent COL2A1 mutations with analysis of genotype-phenotype J Med Genet: first published as 10.1136/jmg.37.4.263 on 1 April 2000. Downloaded from correlation in patients with a lethal type II collagen disorder Geert R Mortier, MaryAnn Weis, Lieve Nuytinck, Lily M King, Douglas J Wilkin, Anne De Paepe, Ralph S Lachman, David L Rimoin, David R Eyre, Daniel H Cohn Abstract osteoarthrosis and little or no skeletal growth Achondrogenesis II-hypochondrogenesis abnormality.2 Achondrogenesis II-hypochond- and severe spondyloepiphyseal dysplasia rogenesis and lethal spondyloepiphyseal dys- congenita (SEDC) are lethal forms of plasia congenita (SEDC) represent the more dwarfism caused by dominant mutations severe end of the spectrum.1 5–7 These entities in the type II collagen gene (COL2A1). To are characterised by severe disproportionate identify the underlying defect in seven short stature of prenatal onset. The distinction cases with this group of conditions, we between these phenotypes is mainly based used the combined strategy of cartilage upon clinical, radiographic, and morphological protein analysis and COL2A1 mutation features but considerable phenotypic overlap 1 6–8 analysis. Overmodified type II collagen often hampers proper classification. and the presence of type I collagen was All of the previously reported cases of found in the cartilage matrix of all seven achondrogenesis II-hypochondrogenesis in which the molecular defect was found were Department of cases. Five patients were heterozygous for Medical Genetics, a nucleotide change that predicted a heterozygous for a mutation in the COL2A1 University Hospital of glycine substitution in the triple helical gene resulting in a glycine substitution in the Gent, De Pintelaan triple-helical domain of the proá1(II) collagen domain (G313S, G517V, G571A, G910C, 9–16 185, B-9000 Gent, G943S). -

Skeletal Dysplasia Panel Versie V1 (345 Genen) Centrum Voor Medische Genetica Gent
H9.1-OP2-B40: Genpanel Skeletal dysplasia, V1, in voege op 14/02/2020 Skeletal_dysplasia panel versie V1 (345 genen) Centrum voor Medische Genetica Gent Associated phenotype, OMIM phenotype ID, phenotype Gene OMIM gene ID mapping key and inheritance pattern Atrial fibrillation, familial, 12, 614050 (3), Autosomal dominant; ABCC9 601439 Cardiomyopathy, dilated, 1O, 608569 (3); Hypertrichotic osteochondrodysplasia, 239850 (3), Autosomal dominant Congenital heart defects and skeletal malformations syndrome, 617602 (3), Autosomal dominant; Leukemia, Philadelphia ABL1 189980 chromosome-positive, resistant to imatinib, 608232 (3), Somatic mutation Short stature and advanced bone age, with or without early-onset osteoarthritis and/or osteochondritis dissecans, 165800 (3), ACAN 155760 Autosomal dominant; Spondyloepimetaphyseal dysplasia, aggrecan type, 612813 (3), Autosomal recessive; ?Spondyloepiphyseal dysplasia, Kimberley type, 608361 (3), Autosomal dominant Spondyloenchondrodysplasia with immune dysregulation, 607944 ACP5 171640 (3), Autosomal recessive Fibrodysplasia ossificans progressiva, 135100 (3), Autosomal ACVR1 102576 dominant Weill-Marchesani syndrome 1, recessive, 277600 (3), Autosomal ADAMTS10 608990 recessive Weill-Marchesani 4 syndrome, recessive, 613195 (3), Autosomal ADAMTS17 607511 recessive ADAMTSL2 612277 Geleophysic dysplasia 1, 231050 (3), Autosomal recessive AFF4 604417 CHOPS syndrome, 616368 (3), Autosomal dominant AGA 613228 Aspartylglucosaminuria, 208400 (3), Autosomal recessive Rhizomelic chondrodysplasia punctata,