Study of the Effect of Acid Atmospheres in Solar Reflectors Durability Under Accelerated Aging Conditions
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Advances in Concentrating Solar Thermal Research and Technology Related Titles
Advances in Concentrating Solar Thermal Research and Technology Related titles Performance and Durability Assessment: Optical Materials for Solar Thermal Systems (ISBN 978-0-08-044401-7) Solar Energy Engineering 2e (ISBN 978-0-12-397270-5) Concentrating Solar Power Technology (ISBN 978-1-84569-769-3) Woodhead Publishing Series in Energy Advances in Concentrating Solar Thermal Research and Technology Edited by Manuel J. Blanco Lourdes Ramirez Santigosa AMSTERDAM • BOSTON • HEIDELBERG LONDON • NEW YORK • OXFORD • PARIS • SAN DIEGO SAN FRANCISCO • SINGAPORE • SYDNEY • TOKYO Woodhead Publishing is an imprint of Elsevier Woodhead Publishing is an imprint of Elsevier The Officers’ Mess Business Centre, Royston Road, Duxford, CB22 4QH, United Kingdom 50 Hampshire Street, 5th Floor, Cambridge, MA 02139, United States The Boulevard, Langford Lane, Kidlington, OX5 1GB, United Kingdom Copyright © 2017 Elsevier Ltd. All rights reserved. No part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, recording, or any information storage and retrieval system, without permission in writing from the publisher. Details on how to seek permission, further information about the Publisher’s permissions policies and our arrangements with organizations such as the Copyright Clearance Center and the Copyright Licensing Agency, can be found at our website: www.elsevier.com/permissions. This book and the individual contributions contained in it are protected under copyright by the Publisher (other than as may be noted herein). Notices Knowledge and best practice in this field are constantly changing. As new research and experience broaden our understanding, changes in research methods, professional practices, or medical treatment may become necessary. -

Efficiency Gain of the Solar Trough Receiver Using Different Optically Active Layers
EFFICIENCY GAIN OF THE SOLAR TROUGH RECEIVER USING DIFFERENT OPTICALLY ACTIVE LAYERS Khaled Shehata Baiuomy Mohamad Supervised by: Professor Philippe Ferrer University of Witwatersrand Johannesburg, 24th October 2019 A dissertation submitted for the fulfillment of the requirements of the degree of Doctor of Philosophy to the Faculty of Science, University of Witwatersrand i Declaration I declare that this thesis is my own, unaided work. It is being submitted for the Degree of Doctor of Philosophy at the University of the Witwatersrand, Johannesburg. It has not been submitted before for any degree or examination at any other University. Student: Khaled Shehata Baiuomy Mohamad Supervisor: Professor Philippe Ferrer ii Dedication To my family. iii Presentations arising from this study Paper Presentation, “Thermal performance analysis of novel alternative designs for parabolic trough solar collector,” 64th Annual Conference of the South African Institute of Physics (July 2019). (Granted an award for the best Ph.D. oral presentation in Applied Physics division) Paper Presentation, “Experimental and numerical study of a cavity and hot mirror receiver of the parabolic trough collector,” 63rd Annual Conference of the South African Institute of Physics (June 2018). Invited presentation, “Parabolic trough Efficiency gain through the use of a cavity absorber with a hot mirror,” Material and Energy research group, workshop, (November 2017). Invited Presentation, “Experimental and Numerical Heat Transfer Analysis of Cavity absorber and The Application of Different Optically Active Layer for Parabolic Solar Trough Concentrator,” Physics school, Wits University, (October 2017). Paper Presentation, “Experimental and Numerical Heat Transfer Analysis of Cavity absorber and The Application of Different Optically Active Layer for Parabolic Solar Trough Concentrator,” 62nd Annual Conference of the South African Institute of Physics (July 2017). -

3M Solar Technologies
3M Film Technologies Durable Films for Solar Light Management Tim Hebrink Staff Scientist 3M Company March 28, 2012 Photo courtesy of Ray Colby with Sundial Solar “Creating a brighter, more durable, more secure, and cleaner future” Hebrink Residence – Scandia Regional Lab 3M Film Technologies c-Si 3M Strategy for Solar Fabrication Reduced costs Thin Film Installation Cost Conversion kWh CPV Efficiency Light Management Increased energy Encapsulation output CSP Thermal management 3M Film Technologies 3M Solar Light Management Technologies Platforms Products Market Segments SMF 1100 Concentrated CSP Solar Power Electricity Generation Light Multilayer Industrial / Building Concentration Mirror Films Solar Thermal Heating Structural Low X Concentrated Panels CPV PhotoVoltaic Electricity Generation PhotoVoltaic and Surface Flat Panel Solar Thermal Structured Films/coatings Light Capture Weatherable Front Anti-Soil Thin Film and Back Surface Coatings Films Films Tapes Adhesives 3M Films/Tapes/Adhesives for c-Si Photovoltaic Junction Box Bonding Die cut of 3M™ Solar Acrylic Foam Tape or Bead of 3M™ PV 1000 Adhesive/Sealant Encapsulating 3M™ Scotchshield™ Film 17T (backside barrier film) Cosmetic Applications 3M™ Specialty Tapes Cell Positioning 3M™ Specialty Tapes Frame Bonding 3M™ Solar Acrylic Foam Tape Identification Solution 3M™ Performance Label Materials © 3M 2009. All Rights Reserved. 3M Film Technologies Multi-layer Optical Mirror Films ¼ Wave Constructive Interference Reflected waves – 100-1000 layers – 15-200 nm thick Incident wave – -
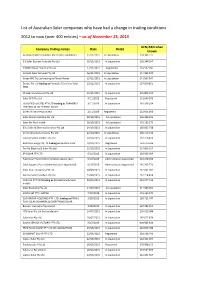
List of Australian Solar Companies Who Have Had a Change in Trading Conditions
List of Australian Solar companies who have had a change in trading conditions 2012 to now (over 400 entries) – as of November 25, 2015 ACN/ABN when Company trading names Date Detail known Australian Micro Inverters Pty Ltd (In Liquidation) 25/11/2015 In Liquidation 159 386 777 S.X Solar Express Australia Pty Ltd 19/11/2015 In Liquidation 126 348 507 CAIRNS VALUE SOLAR PTY LTD 17/11/2015 Registered 154 587 185 Armada Solar Services Pty Ltd 16/11/2015 In Liquidation 144 965 042 Green RRC Pty Ltd trading as Planet Power 13/11/2015 In Liquidation 141 584 947 Dorost Pty Ltd trading as Formerly T/as Clear Solar 12/11/2015 In Liquidation 137 609 851 NSW JP Solar Installations Pty Ltd 12/11/2015 In Liquidation 155 806 125 Solar DFO Pty Ltd 4/11/2015 Registered 151 646 203 QUALIFIED QUOTES PTY LTD trading as FORMERLY 3/11/2015 In Liquidation 165 356 294 TRADING AS NO DEPOSIT SOLAR Green Alliance Pty Limited 2/11/2015 Registered 122 821 363 Solar Winds Australia Pty Ltd 30/10/2015 In Liquidation 126 384 325 Solar Set Pty Limited 28/10/2015 In Liquidation 145 163 579 Elite Solar & Electrical Services Pty Ltd 24/10/2015 In Liquidation 166 865 738 Green Initiatives Services Pty Ltd 22/10/2015 In Liquidation 166 114 512 Sunrise Solar Installers Pty Ltd 20/10/2015 In Liquidation 132 218 850 Red Kite Energy Pty Ltd trading as Red Kite Solar 19/10/2015 Registered 163 525 344 Pro-Ro Electrics & Solar Pty Ltd 13/10/2015 In Liquidation 143 600 337 EP SOLAR PTY LTD 6/10/2015 In Liquidation 138 556 304 Free Solar Pty Ltd (Administrators Appointed) 6/10/2015 Administrators Appointed 131 248 336 Solar Support Pty Ltd (Administrators Appointed) 6/10/2015 Administrators Appointed 145 705 773 Solar City Enterprises Pty Ltd 30/09/2015 In Liquidation 142 641 281 Sunrise Solar Installers Pty Ltd 25/09/2015 In Liquidation 132 218 850 ACN 151 277 913 trading as PH Electrical & Solar 23/09/2015 In Liquidation 151 277 913 Installs Solar Economy Pty Ltd 14/09/2015 In Liquidation 144 963 959 SOLAR SET PTY LIMITED 7/09/2015 In Liquidation 145 163 579 SSW GROUP HOLDINGS PTY. -

REVIEW of RENEWABLE SOLAR ENERGY a Project Presented to The
REVIEW OF RENEWABLE SOLAR ENERGY A Project Presented to the faculty of the Department of Mechanical Engineering California State University, Sacramento Submitted in partial satisfaction of the requirements for the degree of MASTER OF SCIENCE in Mechanical Engineering by Usha Kiranmayee Bhamidipati SUMMER 2012 REVIEW OF RENEWABLE SOLAR ENERGY A Project by Usha Kiranmayee Bhamidipati Approved by: __________________________________, Committee Chair Dr. Dongmei Zhou ___________________________ Date ii Student: Usha Kiranmayee Bhamidipati I certify that this student has met the requirements for format contained in the University format manual, and that this project is suitable for shelving in the Library and credit is to be awarded for the thesis. __________________________, Graduate Coordinator ___________________ Dr. Akihiko Kumagai Date Department of Mechanical Engineering iii Abstract of REVIEW OF RENEWABLE SOLAR ENERGY by Usha Bhamidipati The major challenge that our planet is facing today is the anthropogenic driven climate changes and its link to our global society’s present and future energy needs. Renewable energy sources are now widely regarded as an important energy source. This technology contributes to the reduction of environmental impact, improved energy security and creating new energy industries. Traditional Fossil fuels such as oil, natural gas, coals are in great demand and are highly effective but at the same time they are damaging human health and environment. In terms of environment the traditional fossil fuels are facing a lot of pressure. The most serious challenge would perhaps be confronting the use of coal and natural gas while keeping in mind the greenhouse gas reduction target. It is now clear that in order to keep the levels of CO2 below 550 ppm, it cannot be achieved fundamentally on oil or coal based global economy. -

Polymeric Mirror Films: Durability Improvement and Implementation in New Collector Designs
POLYMERIC MIRROR FILMS: DURABILITY IMPROVEMENT AND IMPLEMENTATION IN NEW COLLECTOR DESIGNS SunShot CSP Program Review 2013 April 23-25 DE-FG36-08GO18027 Awardee: 3M PI: Dr. Raghu Padiyath Presenter: Dr. Daniel Chen Acknowledgment: This material is based upon work supported by the Department of Energy under Award Number DE-FG36- 08GO18027 This report was prepared as an account of work sponsored by an agency of the United States Government. Neither the United States Government nor any agency thereof, nor any of their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process, or service by trade name, trademark, manufacturer, or otherwise does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government or any agency thereof. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States Government or any agency thereof. 1 Outline • Background and Objectives • Technical Approach and Results • Summary and Key Lessons • CSP Cost Reduction and Solar Collectors • Film based Solar Collectors • Conclusions 2 Project Objectives and Outcomes Objectives • Develop novel optical coatings for silvered polymeric mirrors with PMMA front surfaces • Contribute to cost reduction -

Genesis Solar Energy Project PA/FEIS 1 August 2010 Relationship to the Genesis Solar Energy Project Staff Assessment and DEIS
Bureau of Land Management PLAN AMENDMENT/FINAL EIS FOR THE GENESIS SOLAR ENERGY PROJECT Volume 1 of 3 August 2010 DOI Control #: FES 10-42 Publication Index #: BLM/CA/ES-2010-016+1793 NEPA Tracking # DOI-BLM-CA-060-0010-0015-EIS ,,..--...... United States Department ofthe Interior _.... _-- Bureau ofLand Management 1201 Bird Center Drive Palm Springs, CA 92262 Phone (760) 833·7100 IFax (760) 833-7199 http://www.blm.gov/calpalmsprings/ In reply refer to: CACA 048880 August 27, 20 I0 Dear Reader: Enclosed is the Proposed Resource Management Plan-Amendment/Final Environmental Impact Statement (PAIFEIS) for the California Desert Conservation Area (COCA) Plan and Genesis Solar Energy Project (GSEP). The Bureau of Land Management (BLM) prepared the PAiFEIS in consultation with cooperating agencies, taking into account public comments received during the National Environmental Policy Act (NEPA) process. The proposed decision on the plan amendment would add the GSEP site to those identified in the current COCA Plan, as amended, for solar energy production. The preferred alternative on the GSEP is to approve the dry cooling alternative to the right-of-way grant applied for by Genesis Solar, LLC. This PAIFEIS for the GSEP has been developed in accordance with NEPA and the Federal Land Policy and Management Act of 1976. The PA is largely based on the preferred alternative in the Draft Resource Management Plan·AmendmentlDraft Environmentallmpact Statement (DRMP-AiDEIS), which was released on April 9, 2010. The PAIFEIS for the GSEP contains the proposed plan and project description, a summary of changes made between the DRMP·AlDEIS and PRMP-AiFEIS, an analysis of the impacts of the decisions, a summary ofwritten comments received during the public review period for the DRMP AlDEIS and responses to comments. -

Development of Optical Concentrator Systems for Directly Solar Pumped Laser Systems
Yasser Abdel-Fattah Abdel-Hadi Development of optical concentrator systems for directly solar pumped laser systems Technical University of Berlin Institute of Optics 2005 D 83 Development of optical concentrator systems for directly solar pumped laser systems von Yasser Abdel-Fattah Abdel-Hadi, M.Sc. aus Kairo, Ägypten Fakultät II - Mathematik und Naturwissenschaften der Technischen Universität Berlin zur Verleihung des akademischen Grades Doktor der Naturwissenschaften Dr.rer.nat genehmigte Dissertation Berlin 2005 D 83 Tag der wissenschaftlichen Aussprache: 22.12.2005 Berichter: Prof. Dr. –Ing. Adalbert Ding Berichter: Prof. Dr.-Ing. Hans Joachim Eichler Vorsitzender: Prof. Dr. Erwin Sedlmayr To the souls of my parents I present this work… They wanted to be happy to see this work finished… But they died before it… I think they are now happy to see it finished… I also present this work to my home country Egypt and to Germany… Yasser Abstract Two new solar directly-pumped solid state laser systems were developed to avoid the disadvantages of the previously developed system. Using the advantages of the Fresnel lenses, a small solar directly-pumped laser system was developed. This system consists of a rectangular Fresnel lens as a primary concentrator, a secondary concentrator and the laser cavity. A slab laser of the type of Nd:YAG laser was firstly used. For this laser, a collimator convex lens was used as a secondary concentrator. Another laser rod of the type of Nd:YVO4 was used. For this laser, a new non-imaging concentrator of the type of Compound Spherical Concentrator (CSC) was used as a secondary concentrator. -

List of 500+ Solar Companies That Have Closed Down, Been Liquidated, Had Administrators Appointed, Or Proposed De-Registration Since 2011
List of 500+ solar companies that have closed down, been liquidated, had administrators appointed, or proposed de-registration since 2011. Premier Solar CARBON REDUCERS Solar Thermal Energy Sovereign Solar Solar Advisory Service Ascot Solar Metal Craft Solar And Solar Powered Products Ekonomix Solar Solutions AUSSIE WIDE SOLAR Solar Consultants Solar Free Solar Sales And Services Simply Solar Solutions Insulation Solar Renewable Premier Solar OPM IMPORTS T.W.Carlson. Electronics & Solar Village Solar Alliance Sew Residential Solar Platinum Solar CC Corporation National Solar Network SOLAR FREEDOM Installations Solarya Polaris Solar Golden Solar Right Solar Solarshop Australia Lifetime Solar AUSTRALIA Delta Solar Systems Solar-Log Australia & New Queensland Solar Baily Designed Engineering Solar Array Solarshop Australia SOLAR POWER WIDE BAY Metro Solar Elongated Array Solar Zealand Seek Solar Solar Broker Australia Territory Solar Clean Management BURNETT KI Solar Energy Solar Satellite Solutions Eastern Solar Technologies Solarflo The Solar Company Solarshop Constructions Clear Solar Remote Area Power Systems Sanctuary Solar Marathon Solar Solar Energy Tech Austech Solar Unleash Solar Solar Synergy Got To Go Solar Great Solar Solutions Solar Catalog Natural Light & Solar AAA Solar Power Bi-Rite Solar & Electrical Upton Eco For Life Solar Solarco Albury YES SOLAR WA Clipso Solar Solar Solutions VICTORIA Solutions Solar Bright Port Macquarie Premier Solar (VIC) Trackers Solarco Wagga WESTERN CABLING Graham Solar Solar Windows 318 Sun Ray -

DOE Solar Energy Technologies Program FY 2006 Annual Report
Solar Thermal R&D Subprogram Overview Frank (Tex) Wilkins, Team Leader, Solar Thermal R&D The Solar Thermal Subprogram comprises two activities: Concentrating Solar Power (CSP) and Solar Heating and Lighting (SH&L). CSP technologies use mirrors to concentrate the sun’s energy up to 10,000 times sunlight to power conventional turbines, heat engines, or other converters to generate electricity. Energy from CSP systems is high-value renewable power, because energy storage and hybrid designs allow it to be provided when most needed. This is particularly important to utilities that need to increase the amount of power available to them during periods of peak demand. The SH&L research activity develops solar technologies that provide hot water for residential and commercial buildings as well as hybrid solar lighting for commercial buildings. Reducing the cost of these two technologies is important to the goal of a reasonably priced zero energy building. R&D sponsored by the Solar Thermal Subprogram is done in collaboration with industry and university partners. Goals and Objectives Concentrating Solar Power CSP systems currently offer solar electricity at about 12¢–14¢/kWh with systems ranging in size from kilowatt-scale to multi-megawatt power plants. A study by the Western Governors’ Association Solar Task Force projected that large CSP systems will be able to produce power at about 5.5¢/kWh with continued R&D and the deployment of 4,000 MW. Our 2012 goal is to reduce the cost of energy from CSP technology to 9¢–11¢/kWh. The 2020 goal is 5¢–7¢/kWh with up to 12 hours of thermal storage. -

Concentrating Solar Power
Concentrating Solar Power The MIT Faculty has made this article openly available. Please share how this access benefits you. Your story matters. Citation Weinstein, Lee A. et al. “Concentrating Solar Power.” Chemical Reviews 115.23 (2015): 12797–12838. As Published http://dx.doi.org/10.1021/acs.chemrev.5b00397 Publisher American Chemical Society (ACS) Version Author's final manuscript Citable link http://hdl.handle.net/1721.1/106513 Terms of Use Article is made available in accordance with the publisher's policy and may be subject to US copyright law. Please refer to the publisher's site for terms of use. Concentrating Solar Power Lee A. Weinstein,1 James Loomis,1,2 Bikram Bhatia,1 David M. Bierman,1 Evelyn N. Wang,1 and Gang Chen*,1 1Department of Mechanical Engineering, Massachusetts Institute of Technology, Cambridge, MA, 02139, USA. 2Department of Mechanical Engineering, University of Auckland, Auckland 1010, New Zealand. Table of Contents 1. INTRODUCTION ................................................................................................................................ 3 1.1. CSP Configurations ...................................................................................................................... 6 1.2. Maximum efficiency of a CSP system ........................................................................................ 13 2. CONCENTRATOR/REFLECTOR .................................................................................................... 15 2.1. Concentrator characteristics ....................................................................................................... -

Energy, Power, and Transport
Frontispiece Advanced Lunar Base In this panorama of an advanced lunar base, the main habitation modules in the background to the right are shown being covered by lunar soil for radiation protection. The modules on the far right are reactors in which lunar soil is being processed to provide oxygen. Each reactor is heated by a solar mirror. The vehicle near them is collecting liquid oxygen from the reactor complex and will transport it to the launch pad in the background, where a tanker is just lifting off. The mining pits are shown just behind the foreground figure on the left. The geologists in the foreground are looking for richer ores to mine. Artist: Dennis Davidson NASA SP-509, vol. 2 Space Resources Energy, Power, and Transport Editors Mary Fae McKay, David S. McKay, and Michael B. Duke Lyndon B. Johnson Space Center Houston, Texas 1992 National Aeronautics and Space Administration Scientific and Technical Information Program Washington, DC 1992 For sale by the U.S. Government Printing Office Superintendent of Documents, Mail Stop: SSOP, Washington, DC 20402-9328 ISBN 0-16-038062-6 Technical papers derived from a NASA-ASEE summer study held at the California Space Institute in 1984. Library of Congress Cataloging-in-Publication Data Space resources : energy, power, and transport / editors, Mary Fae McKay, David S. McKay, and Michael B. Duke. x, 174 p. : ill. ; 28 cm.—(NASA SP ; 509 : vol. 2) 1. Outer space—Exploration—United States. 2. Natural resources. 3. Space industrialization—United States. I. McKay, Mary Fae. II. McKay, David S. III. Duke, Michael B.