Coordination Chemistry III: Electronic Spectra
Total Page:16
File Type:pdf, Size:1020Kb
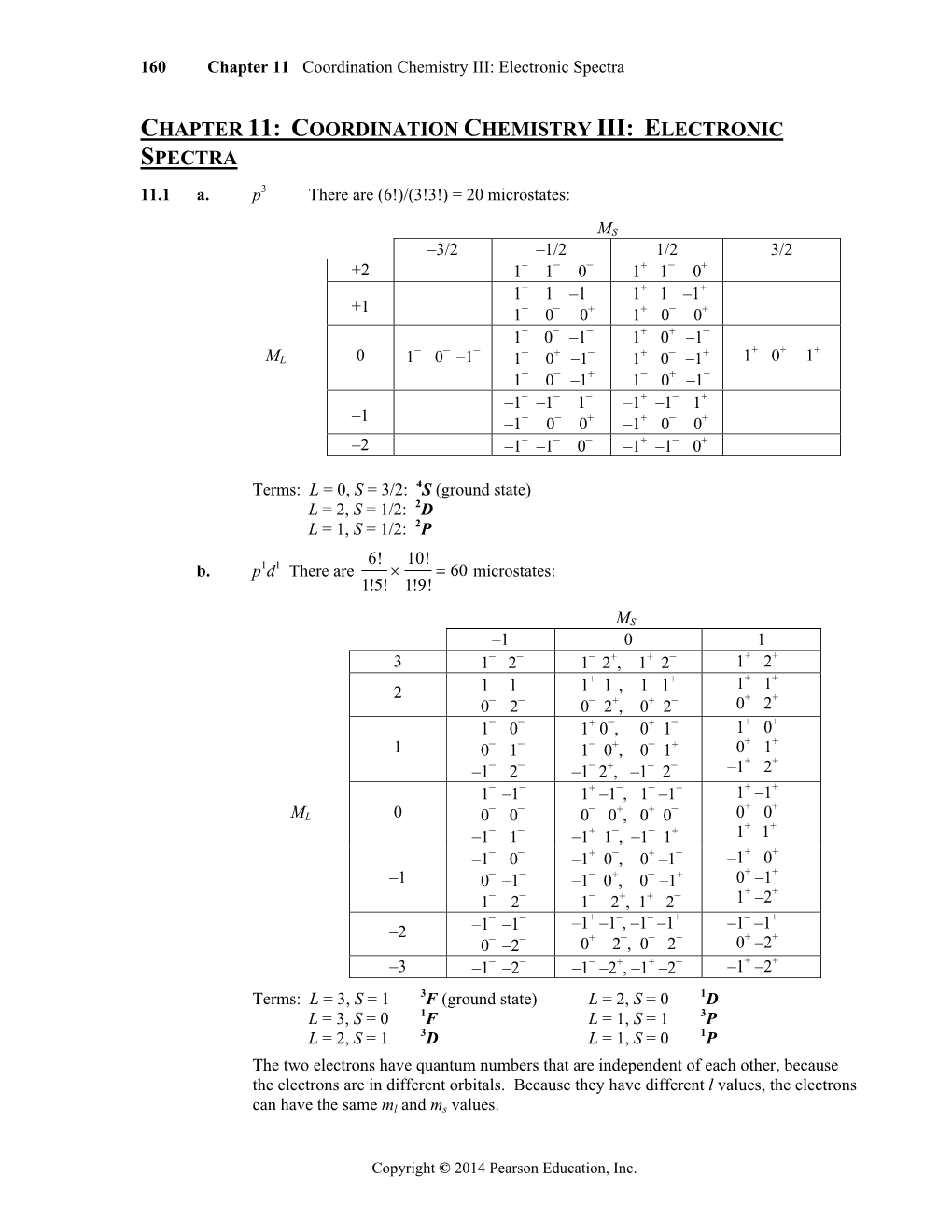
Load more
Recommended publications
-

Review Sheet on Determining Term Symbols
Review Sheet on Determining Term Symbols Term symbols for electronic configurations are useful not only to the spectroscopist but also to the inorganic chemist interested in understanding electronic and magnetic properties of molecules. We will concentrate on the method of Douglas and McDaniel (p. 26ff); however, you may find other treatments equal or superior to the D & M method. Term symbols are a shorthand method used to describe the energy, angular momentum, and spin multiplicity of an atom in any particular state. The general form is a given as Tj where T is a capital letter corresponding to the value of L (the angular momentum quantum number) and may be assigned as S, P, D, F, G, … for |L| = 0, 1, 2, 3, 4, … respectively. The superscript “a” is called the spin multiplicity and can be evaluated as a = 2S +1 where S is the spin quantum number. The subscript “j” is the numerical value of J, a new quantum number defined as: J = l +S, which corresponds to the total orbital and spin angular momentum of the system. The term symbol 3P is read as triplet – Pee state and indicates that there are two unpaired electrons in a state with maximum orbital angular momentum, L=1. The number of microstates (N) of a system corresponds to the total number of distinct arrangements for “e” number of electrons to be placed in “n” number of possible orbital positions. N = # of microstates = n!/(e!(n-e)!) For a set of p orbitals n = 6 since there are 2 positions in each orbital. -

XSAMS: XML Schema for Atomic, Molecular and Solid Data
XSAMS: XML Schema for Atomic, Molecular and Solid Data Version 0.1.1 Draft Document, January 14, 2011 This version: http://www-amdis.iaea.org/xsams/docu/v0.1.1.pdf Latest version: http://www-amdis.iaea.org/xsams/docu/ Previous versions: http://www-amdis.iaea.org/xsams/docu/v0.1.pdf Editors: M.L. Dubernet, D. Humbert, Yu. Ralchenko Authors: M.L. Dubernet, D. Humbert, Yu. Ralchenko, E. Roueff, D.R. Schultz, H.-K. Chung, B.J. Braams Contributors: N. Moreau, P. Loboda, S. Gagarin, R.E.H. Clark, M. Doronin, T. Marquart Abstract This document presents a proposal for an XML schema aimed at describing Atomic, Molec- ular and Particle Surface Interaction Data in distributed databases around the world. This general XML schema is a collaborative project between International Atomic Energy Agency (Austria), National Institute of Standards and Technology (USA), Universit´ePierre et Marie Curie (France), Oak Ridge National Laboratory (USA) and Paris Observatory (France). Status of this document It is a draft document and it may be updated, replaced, or obsoleted by other documents at any time. It is inappropriate to use this document as reference materials or to cite them as other than “work in progress”. Acknowledgements The authors wish to acknowledge all colleagues and institutes, atomic and molecular database experts and physicists who have collaborated through different discussions to the building up of the concepts described in this document. Change Log Version 0.1: June 2009 Version 0.1.1: November 2010 Contents 1 Introduction 11 1.1 Motivation..................................... 11 1.2 Limitations..................................... 12 2 XSAMS structure, types and attributes 13 2.1 Atomic, Molecular and Particle Surface Interaction Data XML Schema Structure 13 2.2 Attributes .................................... -

Atomic Spectroscopy Summary
ATOMIC SPECTROSCOPY SUMMARY TERM SYMBOLS Because the total angular momentum and energy commute, we can use the angular momentum (L) to label the energy states. Note that electron configurations are ambiguous in that we don’t specify which orbital or which spin each electron has. This means that there are a number of different sets of 푚ℓ and 푚푠 that are consistent with a given configuration. Why are there different energies? Because of electron/electron repulsion and spin-orbit coupling, the ways we can fill the orbitals DO NOT NESCESSARILLY have the same energy. (Remember that we talked about how Hund’s rules come from this. How spin up elelctrons interact less with other spin up electrons in the same subshell.) Components of a Term Symbol: 2푆+1 퐿퐽 RULES FOR TERM SYMBOLS Term Symbols are assigned based on two main rules: 1. Unsöld’s Rule: All filled shells/subshells are ignored when calculating L and S. Basically, all of the electrons will have their contributions to spin and orbital angular momentum canceled by other electrons in the same subshell. 2. “holes”: The state of the “hole” (an empty spot in an orbital where an electron could be but isn’t) is the same as the state of an electron. This means that when we look at a p5 we can pretend it is a p1. This is incredibly useful as it means that instead of 5 electrons to tinker with, you can just act like it is 1. CALCULATING L: L is the Total Angular Momentum L= (sum of ℓ values) ….(smallest positive difference of ℓ values) o 2 s electrons (different shells) L=0+0,…,0-0 L=0 o 1 d electron and 1 p electron L=2+1,…,2-1 L=3, 2, 1 S is the Total Spin S is the total spin quantum number of the electrons in an atom. -

Review of Term Symbols Computation from Multi-Electron Systems for Chemistry Students Jamiu A
S O s Contemporary Chemistry p e s n Acce REVIEW ARTICLE Review of Term Symbols Computation from Multi-Electron Systems for Chemistry Students Jamiu A. Odutola* Department of Physics, Chemistry and Mathematics, Alabama Agricultural and Mechanical University, Normal AL 35762, USA Abstract We have computed the term symbols resulting from the coupling of angular momenta of both spin and orbital from the ground state electron configuration. Configurations of equivalent electrons present challenges and were handled by the group theoretical methods. Terms are the combined values of L and S while the combined values of L, S and J defines the level. Key Words: Terms, Term Symbols, Spectroscopic term symbols, State, Level, Sub-level, Equivalent electrons, Non-equivalent electrons, Orbital angular momentum, Spin angular momentum, Spin-Orbital angular momentum, Russell-Saunders Coupling, LS coupling, j-j coupling and multiplicity. Introduction four quantum numbers (n, l, ml, ms). The first three quantum numbers in the set describes the spatial distribution and the Spectroscopic term symbols are very important in classifying last describes the spin state of the electron. n is the principal the electronic states of atoms and molecules particularly in quantum number and it describes the size or the energy level physical and inorganic chemistry [1-4]. There is a vast amount of the shell (or atom). l is the azimuthal or angular momentum of information on the topic of term symbols and techniques quantum number and it describes the shape of the orbital for obtaining these terms in the literature. Many authors gave or the subshell. m is the magnetic quantum number and it credit to such textbooks as Cotton and Wilkinson [5] as well as l describes the spatial orientation or direction of the orbital. -

Synthesis, Structure and Bonding of Actinide Disulphide Dications in the Gas Phase
Synthesis, structure and bonding of actinide disulphide dications in the gas phase Ana F. Lucena,1 Nuno A. G. Bandeira,2,3,4,* Cláudia C. L. Pereira,1,§ John K. Gibson,5 Joaquim Marçalo1,* 1 Centro de Ciências e Tecnologias Nucleares, Instituto Superior Técnico, Universidade de Lisboa, 2695- 066 Bobadela LRS, Portugal 2 Institute of Chemical Research of Catalonia (ICIQ), Barcelona Institute of Technology (BIST), 16 – Av. Països Catalans, 43007 Tarragona, Spain 3 Centro de Química Estrutural, Instituto Superior Técnico, Universidade de Lisboa, Av. Rovisco Pais 1, 1049-001 Lisboa, Portugal 4 Centro de Química e Bioquímica, Faculdade de Ciências, Universidade de Lisboa, Campo Grande, 1749-016 Lisboa, Portugal 5 Chemical Sciences Division, Lawrence Berkeley National Laboratory, Berkeley, California 94720, USA § Present address: REQUIMTE, Faculdade de Ciências e Tecnologia, Universidade Nova de Lisboa, 2829-516 Caparica, Portugal * Corresponding authors: Nuno A. G. Bandeira, email: [email protected]; Joaquim Marçalo, email: [email protected] Abstract 2+ Actinide disulphide dications, AnS2 , were produced in the gas phase for An = Th and Np by reaction of An2+ cations with the sulfur-atom donor COS, in a sequential abstraction process of two sulfur atoms, as examined by FTICR mass spectrometry. For An = Pu and Am, An2+ ions were unreactive with COS and did not yield any sulphide species. High level multiconfigurational (CASPT2) calculations were performed to assess the structures and 2+ bonding of the new AnS2 species obtained for An = Th, Np, as well as for An = Pu to examine trends along the An series, and for An = U to compare with a previous experimental study and 2+ DFT computational scrutiny of US2 . -

Alkali Metal Spectra
____________________________________________________________________________________________________ Subject Chemistry Paper No and Title 8 and Physical Spectroscopy Module No and Title 8: Alkali metal spectra Module Tag CHE_P8_M8 CHEMISTRY PAPER No. : 8 (PHYSICAL SPECTROSCOPY) MODULE No. : 8 (ALKALI METAL SPECTRA) ____________________________________________________________________________________________________ TABLE OF CONTENTS 1. Learning Outcomes 2. Introduction 3. Multi-electron Atoms 4. Electron Orbitals 5. Alkali Metal Spectra 6. Summary CHEMISTRY PAPER No. : 8 (PHYSICAL SPECTROSCOPY) MODULE No. : 8 (ALKALI METAL SPECTRA) ____________________________________________________________________________________________________ 1. Learning Outcomes In this module, you will study about the failures of the Bohr theory, which led to the formulation of the quantum theory. You will understand how the new theory could explain the fine structure in the spectra of hydrogen and hydrogen-like ions, and how this theory can be extended to atoms which have a single electron in their outermost shell, i.e. the alkali metal atoms. You should also be able to write the term symbols for simple one-electron systems. 2. Introduction Bohr’s model could only explain the line spectra of hydrogen and hydrogen-like ions. However, it did not even attempt to explain the spectra of multi-electron atoms. Even in the case of hydrogen, higher resolution shows that each line is split into a doublet, which the Bohr theory was clearly unable to explain. Clearly, the Bohr theory was inadequate and a better, universally applicable, theory was required. 3. Multi-electron Atoms The problem with multi-electron atoms is that, not only are there several electrons getting attracted to the same nucleus, they are repelling each other as well. This repulsion is much harder to deal with. -

A. General (10 Points) 2 Points Each ___B. Configurations & Term
Your name __________________ Grade this page___ Chem 104A - Midterm I Answer Key closed text, closed notes, no calculators There are 70 total points. General advice - if you are stumped by one problem, move on to finish other problems and come back later if time permits. You may use the whole class period. A. General (10 points) 2 Points Each ____ True or false (Enter T or F on line) next to statement: __F__ 1. A 4pz orbital has two radial nodes and 2 angular nodes. It has one angular node – the xy plane. __F__ 2. If the principal quantum number is 3, the orbital shape quantum number (l) can be 0, 1, 2, 3, or 4. The orbital quantum number can only go as high as n-1=l=2; a 3d orbital. __F__ 3. The ratio of the ionization energy for He+ to H is 2:1. The ionization energy goes as Z2, thus the ratio is 4:1. __F__ 4. The ground state of an atom comes from the term with the lowest multiplicity. highest multiplicity __F__ 5. A proper group always has an identity element and a zero element. no need for a zero element B. Configurations & Term Symbols (10 points) 2 Points Each ____ 1. Write out the lowest energy electronic configuration for elemental V. You can use [Ar] for the closed inner shells. [Ar]4s23d 3 2. Write out the symbol for the lowest energy term (circle the multiplicity): A term is a collection of levels with the same L and S. max MS = 3/2 -> S=3/2; max ML=2+1+0 = 3 -> L=3 -> ‘F’ symbol 2S+1L -> 4F 3. -

Part 1 Copyrighted Material
PART 1 Structures and microstructures COPYRIGHTED MATERIAL 1 The electron structure of atoms number of electrons, each of which carries one unit What is a wavefunction and what information of negative charge. For our purposes, all nuclei can does it provide? be imagined to consist of tightly bound subatomic particles called neutrons and protons, which are together called nucleons. Neutrons carry no charge Why does the periodic table summarise both and protons carry a charge of one unit of positive the chemical and the physical properties of the charge. Each element is differentiated from all elements? others by the number of protons in the nucleus, called the proton number or atomic number, Z.Ina What is a term scheme? neutral atom, the number of protons in the nucleus is exactly balanced by the Z electrons in the outer electron cloud. The number of neutrons in an atomic nucleus can vary slightly. The total number nucleons (protons plus neutrons) defines the mass number, A, of an atom. Variants of atoms that have 1.1 Atoms the same atomic number but different mass numbers are called isotopes of the element. For example, the All matter is composed of aggregates of atoms. element hydrogen has three isotopes, with mass With the exception of radiochemistry and radio- numbers 1, called hydrogen; 2 (one proton and activity (Chapter 16) atoms are neither created nor one neutron), called deuterium; and 3 (one proton destroyed during physical or chemical changes. It and two neutrons), called tritium. An important has been determined that 90 chemically different isotope of carbon is radioactive carbon-14, that atoms, the chemical elements, are naturally present has 14 nucleons in its nucleus, 6 protons and 8 on the Earth, and others have been prepared by neutrons. -

Ground State Nuclear Magnetic Resonance Chemical Shifts Predict Charge-Separated Excited State Lifetimes † † † † † Jing Yang, Dominic K
Article Cite This: Inorg. Chem. 2018, 57, 13470−13476 pubs.acs.org/IC Ground State Nuclear Magnetic Resonance Chemical Shifts Predict Charge-Separated Excited State Lifetimes † † † † † Jing Yang, Dominic K. Kersi, Casseday P. Richers, Logan J. Giles, Ranjana Dangi, † ‡ § § Benjamin W. Stein, Changjian Feng, Christopher R. Tichnell, David A. Shultz,*, † and Martin L. Kirk*, † ‡ Department of Chemistry and Chemical Biology and College of Pharmacy, The University of New Mexico, Albuquerque, New Mexico 87131-0001, United States § Department of Chemistry, North Carolina State University, Raleigh, North Carolina 27695-8204, United States *S Supporting Information ABSTRACT: Dichalcogenolene platinum(II) diimine com- ′ plexes, (LE,E )Pt(bpy), are characterized by charge-separated ′ → dichalcogenolene donor (LE,E ) diimine acceptor (bpy) ligand-to-ligand charge transfer (LL′CT) excited states that lead to their interesting photophysics and potential use in solar energy conversion applications. Despite the intense interest in these complexes, the chalcogen dependence on the lifetime of the triplet LL′CT excited state remains ′ unexplained. Three new (LE,E )Pt(bpy) complexes with mixed chalcogen donors exhibit decay rates that are dominated by a spin−orbit mediated nonradiative pathway, the magnitude of which is proportional to the anisotropic covalency provided by the mixed-chalcogen donor ligand environment. This anisotropic covalency is dramatically revealed in the 13C NMR chemical shifts of the donor carbons that bear the chalcogens and is further probed by S K-edge XAS. Remarkably, the NMR chemical shift differences also correlate with the spin−orbit matrix element that connects the triplet excited state with the ground state. Consequently, triplet LL′CT excited state lifetimes are proportional to both functions, demonstrating that specific ground state NMR chemical shifts can be used to evaluate spin−orbit coupling contributions to excited state lifetimes. -

Bsc Chemistry
Subject Chemistry Paper No and Title Paper 7: Inorganic Chemistry-II (Metal-Ligand Bonding, Electronic Spectra and Magnetic Properties of Transition Metal Complexes) Module No and Title 11, Electronic spectra of coordination complexes III Module Tag CHE_P7_M11 CHEMISTRY Paper 7: Inorganic Chemistry-II (Metal-Ligand Bonding, Electronic Spectra and Magnetic Properties of Transition Metal Complexes) Module 11: Electronic spectra of coordination complexes III TABLE OF CONTENTS 1. Learning Outcomes 2. Introduction 3. Spinning of an electron 4. Orbital motion of an electron 4.1 Defining an orbital 4.2 Orbital 5. Spin-Orbit coupling 4.1 What is spin-orbit coupling? 4.2 Free ion terms 6. Summary CHEMISTRY Paper 7: Inorganic Chemistry-II (Metal-Ligand Bonding, Electronic Spectra and Magnetic Properties of Transition Metal Complexes) Module 11: Electronic spectra of coordination complexes III 1. Learning Outcomes After studying this module, you shall be able to Know the difference between spinning and orbital motion of an electron Learn various factors influencing the spin orbit coupling Analyze various free ion terms for different configurations Understand the orbital in an atom along with orbital motion 2. Introduction For multi-electronic atoms and ions, the spin and orbital motion of various electrons are coupled together by spin-orbit coupling or L-S coupling or Russell-Saunders coupling. In this practice the total angular momentum represented by L and total spin angular momentum given by S, combine together to generate a new quantum number given by J. 3. Spinning of an electron A spherical body rotating about a fixed axis that passes through a point at the center of the sphere represents a model that how electron behaves in an atom. -

A COMPARATIVE STUDY of the ATOMIC TERM SYMBOLS of F3 and F11 CONFIGURATION P
ACPI Acta Chim. Pharm. Indica: 2(1), 2012, 32-45 ISSN 2277-288X A COMPARATIVE STUDY OF THE ATOMIC TERM SYMBOLS OF f3 AND f11 CONFIGURATION P. L. MEENA*, P. K. JAIN, N. KUMAR and K. S. MEENA Department of Chemistry, M. L. V. Govt. College, BHILWARA (Raj.) INDIA (Received : 13.10.2011; Revised : 30.10.2011; Accepted : 31.10.2011) ABSTRACT The term is a particular energy state and term symbol is a label to energy state. The importance of these term symbols has been emphasized in connection with the spectral and magnetic properties of complexes and metal free ions and also provide information about the energy of atomic electrons in orbital’s and total spin, total orbital and grand total momenta of whole atom and electronic configuration. Russell-Saunders (L-S) coupling and j-j coupling schemes are important schemes for determination of terms and term symbols of the atoms and ions of inner transition elements in which electrons are filled in a f sub-shell with azimuthal quantum number 3. The determination of terms and term symbols for fn configuration is very difficult work since there are seven orbital’s in f-sub shell which give large number of microstates. In this proposed work computation is done for calculating all possible terms and term symbols regarding for f3 and f11 configurations without any long tabulation with mental exercise and a comparative study was carried out between the f3 and f11 terms and term symbols. The possible microstates and spectroscopic terms calculated for f3 and f11 configuration (ions M+3) are 364 and 17. -

Relativistic Quantum Chemical Calculations Show That the Uranium Molecule U2 Has a Quadruple Bond Stefan Knecht, Hans Jørgen Aagaard Jensen, Trond Saue
Relativistic quantum chemical calculations show that the uranium molecule U2 has a quadruple bond Stefan Knecht, Hans Jørgen Aagaard Jensen, Trond Saue To cite this version: Stefan Knecht, Hans Jørgen Aagaard Jensen, Trond Saue. Relativistic quantum chemical calculations show that the uranium molecule U2 has a quadruple bond. Nature Chemistry, Nature Publishing Group, 2019, 11 (1), pp.40-44. 10.1038/s41557-018-0158-9. hal-01973244 HAL Id: hal-01973244 https://hal.archives-ouvertes.fr/hal-01973244 Submitted on 28 Jan 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Relativistic Quantum Chemical Calculations Show that the Uranium Molecule U2 Has a Quadruple Bond Stefan Knecht∗,1, a) Hans Jørgen Aa. Jensen,2 and Trond Saue3, 4 1)Laboratorium fur¨ Physikalische Chemie, ETH Zurich,¨ Vladimir-Prelog-Weg 2, CH-8093 Zurich,¨ Schweiz 2)Department of Physics, Chemistry and Pharmacy, University of Southern Denmark, DK-5230 Odense M, Denmark 3)Laboratoire de Chimie et Physique Quantiques, IRSAMC, Universit´ePaul Sabatier Toulouse III, 118 Route de Narbonne, F-31062 Toulouse, France 4)Centre for Advanced Study at the Norwegian Academy of Science and Letters, Drammensveien 78, N-0271 Oslo, Norway a)Electronic mail: [email protected] 1 ABSTRACT Our understanding of the bonding, reactivity and electronic structure of actinides is lagging behind the rest of the periodic table.