Detection of Multidrug Resistant Escherichia Coli in the Ovaries Of
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Lions Clubs International Club Membership Register Summary the Clubs and Membership Figures Reflect Changes As of January 2008
LIONS CLUBS INTERNATIONAL CLUB MEMBERSHIP REGISTER SUMMARY THE CLUBS AND MEMBERSHIP FIGURES REFLECT CHANGES AS OF JANUARY 2008 CLUB CLUB LAST MMR FCL YR OB MEMBERSHI P CHANGES TOTAL IDENT CLUB NAME DIST NBR STATUS RPT DATE NEW RENST TRANS DROPS NETCG MEMBERSH 7365 026956 ORAN MEDITERRANEE 415 4 12-2007 23 0 0 0 0 0 23 7365 026957 ALGER DOYEN 415 4 12-2007 19 0 0 0 0 0 19 7365 026961 ORAN DOYEN 415 4 12-2007 17 0 0 0 0 0 17 7365 049534 ORAN EL BAHYA 415 4 01-2008 27 0 0 0 -2 -2 25 7365 050684 SIDI-BEL ABBES 415 7 12-2007 10 0 0 0 -4 -4 6 7365 056315 SIDI BEL ABBES L'ESPOIR 415 7 12-2007 8 0 0 0 0 0 8 7365 058492 ALGER MEDITERNNEE 415 4 01-2008 16 0 0 0 -1 -1 15 7365 058675 ORAN EL MURDJADJO 415 4 01-2008 21 0 0 0 -4 -4 17 7365 059183 SIDI BEL ABBES SOLEIL 415 7 01-2008 14 0 0 0 0 0 14 7365 059444 ALGER CASBAH 415 7 01-2008 9 1 0 0 0 1 10 7365 059566 ORAN BEL AIR 415 4 11-2007 13 1 0 0 -1 0 12 7365 060841 ALGER LA CITADELLE 415 4 01-2008 16 3 0 0 -1 2 18 7365 061031 ORAN PHOENIX 415 4 12-2007 16 2 0 0 0 2 18 7365 061288 ALGER EL GHALIA 415 4 11-2007 9 1 0 0 0 1 10 7365 062921 ALGER MEZGHENA 415 7 12-2007 13 0 0 0 0 0 13 7365 066380 TIZI OUZOU DJURDJURA 415 7 12-2007 15 0 0 0 0 0 15 7365 066657 TIZI OUZOU L'OLIVIER 415 7 12-2007 11 0 0 0 0 0 11 7365 066658 BLIDA EL MENARA 415 7 12-2007 8 4 0 0 -1 3 11 7365 078091 ALGER LUMIERE 415 4 01-2008 15 0 0 0 -2 -2 13 7365 082253 ALGER ZIRI 415 4 01-2008 35 3 0 1 -7 -3 32 7365 082853 SIDI BEL ABBES YASMINE 415 4 12-2007 12 0 0 0 -3 -3 9 7365 088289 SIDI BEL ABBES RAIHAN 415 7 12-2007 7 0 0 0 0 0 7 -

Boa Final4.Pdf
Plenary Talks XXIst Century Perspectives on the Micro & Macro Worlds Jamal Mimouni1 1 Laboratoire de Physique Mathématique et Subatomique, Constantine-1 University e-mail: [email protected] We present a broad perspective on our (Early) XXIth century understanding of both the macro and micro worlds, and whether the dream embodied in the symbolism of Glashow’s snake has been implemented to some degree or recessed away. Do we have a unified vision of the world at those extreme scales? What about the status of that «21 st century» theory «that fell accidentally into the 20th century.” to quote E.Witten, and which is the «ideological» cement of very powerful aspiring-to-be paradigms like Cosmic Inflation, Multiverses, black holes… as well as the inspiration of most pre-Big Bang theories. There is no question that the failure of physics to solve those big puzzles left over from XXth century physics is really stemming from the limitations of experimental particle physics as it stumbles on the «not high enough» energy limit of present day colliders which has put every beyond the Standard Model theory basically on hold. The hints we get from experiments are too feeble and we can’t probe a high enough mass region where are possibly lurking other non-standard gauge bosons and Higgs, not talking about the SUSY zoo, ED particles and other exotic brands. Thus the great hope pinned on the UHE Cosmic Rays for their capability to cross the many orders of magnitude energy gap and reach for the physics of cosmic accelerators and whatever they might be spitting out of new exotic massive particles. -

Language As a Marker of Identity in Tiaret Speech Community
Linguistics and Literature Studies 7(4): 121-125, 2019 http://www.hrpub.org DOI: 10.13189/lls.2019.070401 Language as a Marker of Identity in Tiaret Speech Community Brahmi Mohamed*, Mahieddine Rachid, Bouhania Bachir Department of English, Faculty of Arts and Languages, University of Adrar, Algeria Copyright©2019 by authors, all rights reserved. Authors agree that this article remains permanently open access under the terms of the Creative Commons Attribution License 4.0 International License Abstract This paper explores the linguistic behavior in In his analysis of British English speakers who reside in relation to the identity of speakers who stay in their the USA, Trudgill suggests that how, the extent to which, hometown and speakers who travel from one dialect region and why accommodation to American English occurs to another. Following the methodology of sociolinguistic appears to be due to several factors such as phonological variation studies, combined with qualitative analyses, this contrast and strength of stereotyping. Adopting insights study examines two noticeable linguistic features of Tiaret from these researchers, as well as taking language compared to those acquired by speakers who moved to ideologies into consideration, this study suggests factors other dialect areas. Qualitative analyses of speakers’ social that trigger or fail to trigger accommodation by speakers identities, attitudes and language practices match migrating to a new dialect area. quantitative analyses of patterns of phonological variation. Previous sociolinguistic research has detailed how The study finds that the migrant groups do make changes historical, political and sociolinguistic factors have in their linguistic production due to their continuous influenced issues of language, identity, and conflicts in exposure to a new dialect. -

Administering Vaccination in Interwar Algeria, Author Accepted Version
Clark, H.-L. (2016) Administering vaccination in interwar Algeria: medical auxiliaries, smallpox, and the colonial state in the Communes mixtes. French Politics, Culture and Society, 34(2), pp. 32- 56. (doi:10.3167/fpcs.2016.340203) This is the author’s final accepted version. There may be differences between this version and the published version. You are advised to consult the publisher’s version if you wish to cite from it. http://eprints.gla.ac.uk/147771/ Deposited on: 12 September 2017 Enlighten – Research publications by members of the University of Glasgow http://eprints.gla.ac.uk Administering Vaccination in Interwar Algeria: Auxiliaires médicaux, Smallpox, and the Colonial State in the Communes mixtes Hannah-Louise Clark Trinity College, University of Oxford It is a rain-soaked November afternoon in the city of Constantine in eastern Algeria. I am ensconced in the regional archives, searching for records relating to colonial-era disease control in Algeria’s communes mixtes (mixed communes). In place from 1858 to 1956, these colonial administrative units covered immense swathes of rural territory, encompassing centres de colonisation inhabited by a “mixed” population and outlying Muslim villages and settlements—the douars—under the sole charge of a centrally appointed administrator.1 In one archival box relating to the arrondissement of Bougie (Bejaïa), I find an improvised booklet constructed from quadrille paper threaded together with string. Sloping cursive lettering on the title page proclaims this to be a vaccination logbook: “Year 1936. Protection of Public Health (decree of 27 May 1907). Service of vaccination and revaccination. Mr AMRANE Mohand, vaccinator.” I immediately recognise Mohand ould Ramdan Amrane as one of the auxiliaires médicaux (medical auxiliaries), also known as adjoints techniques de la Santé publique, whose careers I have been tracking through personnel files and correspondence in the Algerian National Archives. -

NAF SHUTTLE NEWSLETTER Week 03|2017
NAF SHUTTLE NEWSLETTER Week 03|2017 Marseilles, January 19th 2017 Newsletter NAF France week 34 NEWSLETTER NAF SHUTTLE WEEK 03 MARSEILLES, JANUARY 19 TH Chers Clients, La Ligne SSL MED est heureuse de vous faire parvenir sa Gazette hebdomadaire de la semaine 03. Vous trouverez ci-après les horaires et rotations de nos services hebdomadaires sur le Maghreb au départ de Marseille sur l’Algérie, la Tunisie et le Maroc. Cordialement, Short Sea Lines MED Dear Customer, SSL MED line is pleased to send you its newsletter, week 03 You’ll find out the schedule and rotations of our weekly services calling Algeria, Tunisia, and Morocco from port of loading Marseilles. Best regards, Short Sea Lines MED NEWSLETTER NAF SHUTTLE WEEK 03 MARSEILLES, JANUARY 19TH Dear Customers, Chers Clients, Thanks to note closings as below: Nous vous remercions de bien vouloir respecter les clôtures ci-dessous Clôture VGM : Vendredi 12h VGM : Friday 12h Clôture BAS : Vendredi 14h Customs: Friday 14h Pour les arrivées par train , merci de bien vouloir créer vos AMQ avec For containers arriving by rail, and to limit impact of late arrivals, please do reconnaissance et BAET Implicite afin de limiter l’impact de retards éventuels. your customs formalities by anticipation. Only theses bookings will be Les cases AP+ « arriv tpt » seront cochées pour vous permettre de réaliser ces opérations. Seuls ces dossiers pourront être maintenus sur liste. maintained on loading list. Pour raison opérationnelle , nous ne pourrons pas étendre les clôtures au-delà For operational reasons, -

International Conference on the Black Arts Movement in the United States and Algeria November 18-19, 2019
Session Six: 11.10-12.10 (Room 35) Emily Jane O’Dell (Yale Law School-Yale University- People’s Democratic Republic of USA), Associate Professor at Sichuan University- Algeria Frantz Fanon: Algeria’s Adoptive Son Pittsburgh Institute Excavating Memories of the 1969 Pan-African Festival: Ministry of Higher Education and Moderator: Nadjiba Bouallegue (University of 08 Mai The Impact of Algeria on Theatre Artists§Writers Scientific Research 1945 -Guelma-Algeria) Mohammed Senoussi (University of M’sila-Algeria), Abdelhamid Ibn Badis University Stéphanie Melyon-Reinette (Pointe-à-Pitre-Réunion) Displacement, Identity and the Syndrome of Hybridity in - Mostaganem Faculty of Foreign Languages Algiers, 1969, The Poetic Mecca: Amiri Baraka, Frantz Chimamanda gozi Adichie’s The Thing Around Your Neck Fanon, Sonny Rupaire& The Revolutionary Culture Hana Bougherira, (University of Skikda-Algeria), Department of English Mira Hafsi (Mohamed Lamine Debaghine University- From “Flaneur” to a “Stalker”: Reconstructing Identity Sétif-Algeria), Frantz Fanon and the Black Arts in Toni Morrison’s God Help the Child Movement: Some Reflections through Drama Discussion: 12.10-12.20 Oussama Mahboub (University of Sousse-Tunisia), The 1960's Legacy of Black Acculturation between Adoption 12.20-1.20: Lunch and Resistance: Liberal vs. Conservative Moral 1.20-2: Discussion and Prospects (Amphi G) Implications in a Comparative Perspective 2-2.30: Closing Ceremony Discussion: 12-12.20 2.30: Sightseeing International Conference Session Seven: 11.10-12-10 (Room 33) On African-Americans and the Race Question “The fact that I had never seen the Algerian The Black Arts Movement Moderator: Michael A. Antonucci, (Keene State College- Casbah was of no more relevance before this Keene-New Hampshire) unanswerable panorama than the fact that the Mohamed Ben Ali Chaker (University of Skikda- in the United States Algeria) Algerians had never seen Harlem. -

Opportunities for Social Entrepreneurship Cultivation In
Lastly, the combination of vulnerability rankings with opportunity rankings displays a high potential Social entrepreneurship takes many forms; one common definition is to describe it as for investment in social entrepreneurship cultivation in the wilayas of Chlef, Tiaret, Djelfa and Oum el for-profit business initiatives that have, at a minimum, a double bottom line: a finan- Bouaghi. cial bottom line that requires financial sustainability as a core element of the busi- ness, as well as a social and/or environmental bottom line. Both of these elements are of equal importance to the sustainability and health of the business. Social entrepre- neurship has only risen to prominence as a known term and strategy in the past decade or so, primarily 3 5 in a response to traditional development, business and CSR initiatives, as well as government 4 4 1 1 1 5 5 inefficiencies in the provision of services and protection of culture and the environment. 1 5 1 3 4 1 1 3 1 1 1 5 3 5 5 5 2 1 3 3 3 2 2 3 3 1 5 5 4 The Arab World is no stranger to social entrepre- 1 4 4 5 1 neurship initiatives. With burgeoning populations 2 4 and in the wake of the Arab Spring, social entrepre- 3 3 neurship has become the go-to solution for many of the youth - which constitute over 30% of the 1 region’s population - seeking to make meaningful change. Algeria is currently the largest Arab country in terms of size; it is also the second most populous country in the MENA region. -

The Mineral Industry of Algeria in 2008
2008 Minerals Yearbook ALGERIA U.S. Department of the Interior September 2010 U.S. Geological Survey THE MINERAL INDUS T RY OF ALGERIA By Mowafa Taib Algeria is the 2d largest country in terms of land area in limestone, and sand); ArcelorMittal of Luxembourg (iron Africa and the 11th largest country in the world. Its geologic ore, limestone, and aggregate); Cancor Mine Inc. of Canada structures include the Tellian chain, the High Plateaus, the (gold-copper, gold-silver); China Geo Engineering Corp. (gold); Saharan Atlas, the Saharan platform, the Hoggar shield, and the Henan Shaolin Hydro Co. Ltd. (copper, gold, manganese, tin, Eglab massif. In 2008, Algeria was a significant producer and zinc-lead); Poly Sciences and Technology Development Co. Ltd. exporter of natural gas and accounted for 2.8% of the world’s of China (gold); Soalka-White Federal Cement of Canada and natural gas output. The country held 4.5 trillion cubic meters Lebanon (kaolin); and Western Mediterranean Zinc of Australia of natural gas reserves, which was 2.5% of the world’s total (zinc-lead) (Ministry of Energy and Mining, 2009, p. 82). gas reserves. Algeria was a significant producer and exporter of crude oil; in terms of the volume of its crude oil production, Production it was ranked third in Africa after Nigeria and Angola and accounted for 2.2% of the world’s crude oil output. In addition In 2008, Algeria’s crude oil production decreased slightly to hydrocarbons, Algeria produced substantial quantities of (by 2.6%) and natural gas output increased modestly (by 1.5%) metal, including gold, iron ore, silver, and steel; industrial compared with 2007 output levels. -

Characterization of Quinolone-Resistant Enterobacteriaceae Strains Isolated from Poultry in Western Algeria: First Report of Qnrs in an Enterobacter Cloacae
Veterinary World, EISSN: 2231-0916 RESEARCH ARTICLE Available at www.veterinaryworld.org/Vol.11/April-2018/10.pdf Open Access Characterization of quinolone-resistant Enterobacteriaceae strains isolated from poultry in Western Algeria: First report of qnrS in an Enterobacter cloacae Qada Benameur1,2, Hassiba Tali-Maamar3, Farida Assaous3, Badia Guettou3, Meki Boutaiba Benklaouz4, Kheira Rahal3 and Meriem-Hind Ben-Mahdi2,5 1. Faculty of Natural Sciences and Life, Abdelhamid Ibn Badis University, Mostaganem, Algeria; 2. Research Laboratory, Health and Animal Production, Higher National Veterinary School, Algiers, Algeria; 3. Medical Bacteriology Laboratory, Pasteur Institute of Algeria, Algiers, Algeria; 4. Veterinary Sciences Institute, Ibn Khaldoun University, Tiaret, Algeria; 5. Higher School of Food Sciences and Agro-alimentary Industries, Algiers, Algeria. Corresponding author: Qada Benameur, e-mail: [email protected] Co-authors: HT: [email protected], FA: [email protected], BG: [email protected], MBB: [email protected], KR: [email protected], MHB: [email protected] Received: 21-12-2017, Accepted: 06-03-2018, Published online: 12-04-2018 doi: 10.14202/vetworld.2018.469-473 How to cite this article: Benameur Q, Tali-Maamar H, Assaous F, Guettou B, Boutaiba Benklaouz M, Rahal K, Ben-Mahdi MH (2018) Characterization of quinolone-resistant Enterobacteriaceae strains isolated from poultry in Western Algeria: First report of qnrS in an Enterobacter cloacae, Veterinary World, 11(4): 469-473. Abstract Aim: Multidrug-resistant (MDR) Enterobacteriaceae have frequently been reported, in both human and veterinary medicine, from different parts of the world as a consequence of antibiotic usage. However, there is a lack of published data regarding antimicrobial resistance in non-Escherichia coli (E. -

Immigration and Refugee Board of Canada Page 1 of 6
Responses to Information Requests - Immigration and Refugee Board of Canada Page 1 of 6 Immigration and Refugee Board of Canada Home > Research Program > Responses to Information Requests Responses to Information Requests Responses to Information Requests (RIR) respond to focused Requests for Information that are submitted to the Research Directorate in the course of the refugee protection determination process. The database contains a seven- year archive of English and French RIRs. Earlier RIRs may be found on the UNHCR's Refworld website. Please note that some RIRs have attachments which are not electronically accessible. To obtain a PDF copy of an RIR attachment, please email the Knowledge and Information Management Unit. 29 November 2013 DZA104676.FE Algeria: Forced marriages, including state protection and resources provided to women who try to avoid a marriage imposed on them; amendments made to the Family Code in 2005 (2011-November 2013) Research Directorate, Immigration and Refugee Board of Canada, Ottawa 1. Forced Marriages According to Voyage.gc.ca, the Government of Canada site that provides information to Canadians travelling or living abroad (Canada 27 June 2013), [translation] "forced marriages have occurred" in Algeria (ibid. 22 Mar. 2013). According to the French daily newspaper Le Figaro, the prevalence of forced marriages in countries of the Maghreb is difficult to assess (30 Nov. 2012). BALSAM, the national network of call centres for victims of violence against women in Algeria, which was founded in 2009 and had 15 call centres throughout Algeria as of 2012, states in its fourth report, published in May 2012, that since the network was implemented, of the 828 women who have contacted the call centres, 12 were victims of forced marriage and 18 were victims of attempted forced marriage (BALSAM May 2012, 4, 7, 27). -
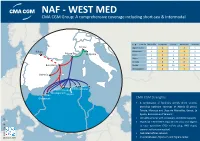
NAF - WEST MED CMA CGM Group: a Comprehensive Coverage Including Short-Sea & Intermodal
NAF - WEST MED CMA CGM Group: A comprehensive coverage including short-sea & Intermodal To From Marseilles La Spezia Genoa Barcelona Valencia Genoa Algiers ODCY 2 4 5 4 5 Bilbao Ghazaouet 2 4 5 4 5 Marseilles La Spezia Oran 2 4 5 4 5 Bejaia 6 8 9 8 9 Annaba 6 8 9 8 9 Barcelona Skikda 7 9 10 9 10 Mostaganem 2 4 5 4 5 Valencia Tunis Skikda Annaba Algiers Rouiba Mostaganem Oran Bejaia Ghazaouet CMA CGM Strengths • A combination of fixed-day weekly direct services providing optimum coverage of Algeria (8 ports), Tunisia, Morocco and Libya via Marseilles, Genoa, La Spezia, Barcelona and Valencia • Versatile services with containers and RORO capacity • Owned ALTERCO ODCY dry port in Rouiba, near Algiers to ease operations (150 reefers plug, IMO depot, scanner, railway connection) • Dedicated offices network www.cma-cgm.com October 2014 • Choice between Algiers dry and Algiers center NAF - WEST MED CMA CGM Group: A comprehensive coverage including short-sea & Intermodal Trieste Venice Genoa Ravenna CMA CGM Contacts Fos sur Mer La Spezia Ancona Livorno General Mailbox Vigo Civitavecchia [email protected] Barcelona Leixoes Naples Salerno Valencia Lisbon Gioia Tauro Bizerte Trapani Skikda Catania Algeciras Algiers Annaba Tunis Bejaia Djen Djen MALTA Oran Sfax TANGIER MED Ghazaouet Casablanca TUNISIA Tripoli Misurata Al Khoms Benghazi Agadir CMA CGM Strengths MOROCCO ALGERIA LIBYA • 9 weekly departures from Marseilles to NAF destinations • Two CMA CGM dedicated hubs: Malta and Tangier Med • Owned and dedicated feeder network from Malta ensuring weekly connections to 18 ports in the whole North Africa • Shuttle services deployed from Tangier Med to Oran, Ghazaouet, Tangier and Casablanca www.cma-cgm.com October 2014. -

Burning the Veil: the Algerian War and the 'Emancipation' of Muslim
3 Unveiling: the ‘revolutionary journées’ of 13 May 1958 Throughout the period from early 1956 to early 1958 putschist forces had been gathering strength both within the army and among right- wing settler organisations and these eventually coalesced on 13 May 1958 when crowds gathered in the Forum and stormed the General Government buildings. The military rapidly used the crisis to effect a bloodless coup and to install a temporary ‘revolutionary’ authority headed by a Committee of Public Safety (Comité de salut public or CSP) under Generals Massu and Salan. There then followed a tense stand- off between the army in Algeria and the new Paris government headed by Pierre Pfl imlin, a three-week period during which civil war was a real possibility, until de Gaulle agreed to assume, once again, the role of ‘saviour of the nation’, and was voted into power by the National Assembly on 1 June.1 ‘13 May’ was one of the great turning points in modern French history, not only because it marked a key stage in the Algerian War, but more signifi cantly the collapse of the Fourth Republic, de Gaulle’s return to power, and the beginnings of the new constitutional regime of the Fifth Republic. The planning of the coup and its implementation was extraordi- narily complex – the Bromberger brothers in Les 13 Complots du 13 mai counted thirteen strands2 – but basically two antagonistic politi- cal formations reached agreement to rally to the call for de Gaulle’s return to power. On the one hand there was a secret plot by Gaullists, most notably Michel Debré (soon to become Prime Minister), Jacques Soustelle, Léon Delbecque and Jacques Chaban-Delmas (acting Minister of Defence), to engineer the return of the General so as to resolve the political crisis of the ‘system’, the dead hand of the party system of the Fourth Republic, which they viewed as destroying the grandeur of France.