Overview of the Current Treatment Strategy in Extranodal NK/T-Cell Lymphoma: from Diagnosis to Recurrence
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Treatment of Localized Extranodal NK/T Cell Lymphoma, Nasal Type: a Systematic Review Seok Jin Kim†, Sang Eun Yoon† and Won Seog Kim*
Kim et al. Journal of Hematology & Oncology (2018) 11:140 https://doi.org/10.1186/s13045-018-0687-0 REVIEW Open Access Treatment of localized extranodal NK/T cell lymphoma, nasal type: a systematic review Seok Jin Kim†, Sang Eun Yoon† and Won Seog Kim* Abstract Extranodal natural killer/T cell lymphoma (ENKTL), nasal type, presents predominantly as a localized disease involving the nasal cavity and adjacent sites, and the treatment of localized nasal ENKTL is a major issue. However, given its rarity, there is no standard therapy based on randomized controlled trials and therefore a lack of consensus on the treatment of localized nasal ENKTL. Currently recommended treatments are based mainly on the results of phase II studies and retrospective analyses. Because the previous outcomes of anthracycline-containing chemotherapy were poor, non- anthracycline-based chemotherapy regimens, including etoposide and L-asparaginase, have been used mainly for patients with localized nasal ENKTL. Radiotherapy also has been used as a main component of treatment because it can produce a rapid response. Accordingly, the combined approach of non-anthracycline-based chemotherapy with radiotherapy is currently recommended as a first-line treatment for localized nasal ENKTL. This review summarizes the different approaches for the use of non-anthracycline-based chemotherapy with radiotherapy including concurrent, sequential, and sandwich chemoradiotherapy, which have been proposed as a first-line treatment for newly diagnosed patients with localized nasal ENKTL. Keywords: Extranodal NK/T cell lymphoma, Chemoradiotherapy, Localized disease Background Treatment for newly diagnosed patients with localized Extranodal natural killer/T cell lymphoma (ENKTL), nasal nasal ENKTL type, is a rare subtype of non-Hodgkin lymphoma [1]. -

Hodgkin Lymphoma Treatment Regimens
HODGKIN LYMPHOMA TREATMENT REGIMENS (Part 1 of 5) Clinical Trials: The National Comprehensive Cancer Network recommends cancer patient participation in clinical trials as the gold standard for treatment. Cancer therapy selection, dosing, administration, and the management of related adverse events can be a complex process that should be handled by an experienced health care team. Clinicians must choose and verify treatment options based on the individual patient; drug dose modifications and supportive care interventions should be administered accordingly. The cancer treatment regimens below may include both U.S. Food and Drug Administration-approved and unapproved indications/regimens. These regimens are provided only to supplement the latest treatment strategies. These Guidelines are a work in progress that may be refined as often as new significant data become available. The NCCN Guidelines® are a consensus statement of its authors regarding their views of currently accepted approaches to treatment. Any clinician seeking to apply or consult any NCCN Guidelines® is expected to use independent medical judgment in the context of individual clinical circumstances to determine any patient’s care or treatment. The NCCN makes no warranties of any kind whatsoever regarding their content, use, or application and disclaims any responsibility for their application or use in any way. Classical Hodgkin Lymphoma1 Note: All recommendations are Category 2A unless otherwise indicated. Primary Treatment Stage IA, IIA Favorable (No Bulky Disease, <3 Sites of Disease, ESR <50, and No E-lesions) REGIMEN DOSING Doxorubicin + Bleomycin + Days 1 and 15: Doxorubicin 25mg/m2 IV push + bleomycin 10units/m2 IV push + Vinblastine + Dacarbazine vinblastine 6mg/m2 IV over 5–10 minutes + dacarbazine 375mg/m2 IV over (ABVD) (Category 1)2-5 60 minutes. -

Ciera L. Patzke, Alison P. Duffy, Vu H. Duong, Firas El Chaer 4, James A
Journal of Clinical Medicine Article Comparison of High-Dose Cytarabine, Mitoxantrone, and Pegaspargase (HAM-pegA) to High-Dose Cytarabine, Mitoxantrone, Cladribine, and Filgrastim (CLAG-M) as First-Line Salvage Cytotoxic Chemotherapy for Relapsed/Refractory Acute Myeloid Leukemia Ciera L. Patzke 1, Alison P. Duffy 1,2, Vu H. Duong 1,3, Firas El Chaer 4, James A. Trovato 2, Maria R. Baer 1,3, Søren M. Bentzen 1,3 and Ashkan Emadi 1,3,* 1 Greenebaum Comprehensive Cancer Center, University of Maryland, Baltimore, MD 21201, USA; [email protected] (C.L.P.); aduff[email protected] (A.P.D.); [email protected] (V.H.D.); [email protected] (M.R.B.); [email protected] (S.M.B.) 2 School of Pharmacy, University of Maryland, Baltimore, MD 21201, USA; [email protected] 3 School of Medicine, University of Maryland, Baltimore, MD 21201, USA 4 School of Medicine, University of Virginia, Charlottesville, VA 22908, USA; [email protected] * Correspondence: [email protected]; Tel.: +1-410-328-2596 Received: 10 January 2020; Accepted: 13 February 2020; Published: 16 February 2020 Abstract: Currently, no standard of care exists for the treatment of relapsed or refractory acute myeloid leukemia (AML). We present our institutional experience with using either CLAG-M or HAM-pegA, a novel regimen that includes pegaspargase. This is a retrospective comparison of 34 patients receiving CLAG-M and 10 receiving HAM-pegA as first salvage cytotoxic chemotherapy in the relapsed or refractory setting. Composite complete response rates were 47.1% for CLAG-M and 90% for HAM-pegA (p = 0.027). -

BC Cancer Benefit Drug List September 2021
Page 1 of 65 BC Cancer Benefit Drug List September 2021 DEFINITIONS Class I Reimbursed for active cancer or approved treatment or approved indication only. Reimbursed for approved indications only. Completion of the BC Cancer Compassionate Access Program Application (formerly Undesignated Indication Form) is necessary to Restricted Funding (R) provide the appropriate clinical information for each patient. NOTES 1. BC Cancer will reimburse, to the Communities Oncology Network hospital pharmacy, the actual acquisition cost of a Benefit Drug, up to the maximum price as determined by BC Cancer, based on the current brand and contract price. Please contact the OSCAR Hotline at 1-888-355-0355 if more information is required. 2. Not Otherwise Specified (NOS) code only applicable to Class I drugs where indicated. 3. Intrahepatic use of chemotherapy drugs is not reimbursable unless specified. 4. For queries regarding other indications not specified, please contact the BC Cancer Compassionate Access Program Office at 604.877.6000 x 6277 or [email protected] DOSAGE TUMOUR PROTOCOL DRUG APPROVED INDICATIONS CLASS NOTES FORM SITE CODES Therapy for Metastatic Castration-Sensitive Prostate Cancer using abiraterone tablet Genitourinary UGUMCSPABI* R Abiraterone and Prednisone Palliative Therapy for Metastatic Castration Resistant Prostate Cancer abiraterone tablet Genitourinary UGUPABI R Using Abiraterone and prednisone acitretin capsule Lymphoma reversal of early dysplastic and neoplastic stem changes LYNOS I first-line treatment of epidermal -

Australian Public Assessment Report for Pegaspargase
Australian Public Assessment Report for pegaspargase Proprietary Product Name: Oncaspar Sponsor: Baxalta Australia September 2018 Therapeutic Goods Administration About the Therapeutic Goods Administration (TGA) • The Therapeutic Goods Administration (TGA) is part of the Australian Government Department of Health and is responsible for regulating medicines and medical devices. • The TGA administers the Therapeutic Goods Act 1989 (the Act), applying a risk management approach designed to ensure therapeutic goods supplied in Australia meet acceptable standards of quality, safety and efficacy (performance) when necessary. • The work of the TGA is based on applying scientific and clinical expertise to decision- making, to ensure that the benefits to consumers outweigh any risks associated with the use of medicines and medical devices. • The TGA relies on the public, healthcare professionals and industry to report problems with medicines or medical devices. TGA investigates reports received by it to determine any necessary regulatory action. • To report a problem with a medicine or medical device, please see the information on the TGA website < https://www.tga.gov.au>. About AusPARs • An Australian Public Assessment Report (AusPAR) provides information about the evaluation of a prescription medicine and the considerations that led the TGA to approve or not approve a prescription medicine submission. • AusPARs are prepared and published by the TGA. • An AusPAR is prepared for submissions that relate to new chemical entities, generic medicines, major variations and extensions of indications. • An AusPAR is a static document; it provides information that relates to a submission at a particular point in time. • A new AusPAR will be developed to reflect changes to indications and/or major variations to a prescription medicine subject to evaluation by the TGA. -

A Phase I Trial of Pemetrexed Plus Gemcitabine Given Biweekly with B-Vitamin Support in Solid Tumor Malignancies Or Advanced Epithelial Ovarian Cancer Martee L
Cancer Therapy: Clinical A Phase I Trial of Pemetrexed Plus Gemcitabine Given Biweekly with B-Vitamin Support in Solid Tumor Malignancies or Advanced Epithelial Ovarian Cancer Martee L. Hensley,1Joseph Larkin,1Matthew Fury,1Scott Gerst,1D. Fritz Tai,2 Paul Sabbatini,1 Jason Konner,1Mauro Orlando,2 Tiana L. Goss,2 and Carol A. Aghajanian1 Abstract Purpose: To determine the maximally tolerated dose (MTD) of biweekly pemetrexed with gemcitabine plus B12 and folate supplementation in patients with advanced solid tumors and ovarian cancer. Experimental Design: Patients with no prior pemetrexed or gemcitabine therapy enrolled in cohorts of three, expanding to six if dose-limiting toxicity (DLT) was observed. Pemetrexed, escalated from to 700 mg/m2, was given before gemcitabine 1,500 mg/m2 every 14 days. DLTs were grade 4 neutropenia lasting >7 days or febrile neutropenia, grade 4 or 3 thrombocytopenia (with bleeding), grade z3 nonhematologic toxicity, or treatment delay of z1 week due to unresolved toxicity. Results: The ovarian cancer cohort enrolled 24 patients with unlimited prior cytotoxic chemo- therapies. MTD was observed at pemetrexed 600 mg/m2, with 2 of 9 patients experiencing DLT. Most common grade 3 to 4 toxicities per patient were neutropenia (83%), leukopenia (67%), lymphopenia (73%), and febrile neutropenia (12%). Median cycle per patient was 8 (range, 1-16). Six of 21 (28%) patients had confirmed partial responses. Study protocol was modified for the solid tumor cohort (n = 30) to enroll patients with two or more prior cytotoxic regimens. MTD was observed at pemetrexed 500 mg/m2, with 1of 9 patients experiencing DLT. -

GEMCITABINE Hcl) for INJECTION
FDA REVISED LABEL – VERSION 082598 Page 1 PA 1634 AMP GEMZAR® (GEMCITABINE HCl) FOR INJECTION DESCRIPTION Gemzar® (gemcitabine HCl) is a nucleoside analogue that exhibits antitumor activity. Gemcitabine HCl is 2'-deoxy-2',2'-difluorocytidine monohydrochloride (ß-isomer). The structural formula is as follows: NH 2•HCl N HO N O O F H OH F The empirical formula for gemcitabine HCl is C9H11F2N3O4 • HCl. It has a molecular weight of 299.66. Gemcitabine HCl is a white to off-white solid. It is soluble in water, slightly soluble in methanol, and practically insoluble in ethanol and polar organic solvents. The clinical formulation is supplied in a sterile form for intravenous use only. Vials of Gemzar contain either 200 mg or 1 g of gemcitabine HCl (expressed as free base) formulated with mannitol (200 mg or 1 g, respectively) and sodium acetate (12.5 mg or 62.5 mg, respectively) as a sterile lyophilized powder. Hydrochloric acid and/or sodium hydroxide may have been added for pH adjustment. CLINICAL PHARMACOLOGY Gemcitabine exhibits cell phase specificity, primarily killing cells undergoing DNA synthesis (S-phase) and also blocking the progression of cells through the G1/S-phase boundary. Gemcitabine is metabolized intracellularly by nucleoside kinases to the active diphosphate (dFdCDP) and triphosphate (dFdCTP) nucleosides. The cytotoxic effect of gemcitabine is attributed to a combination of two actions of the diphosphate and the triphosphate nucleosides, which leads to inhibition of DNA synthesis. First, gemcitabine diphosphate inhibits ribonucleotide reductase, which is responsible for catalyzing the reactions that generate the deoxynucleoside triphosphates for DNA synthesis. -

Intravesical Administration of Therapeutic Medication for the Treatment of Bladder Cancer Jointly Developed with the Society of Urologic Nurses and Associates (SUNA)
Intravesical Administration of Therapeutic Medication for the Treatment of Bladder Cancer Jointly developed with the Society of Urologic Nurses and Associates (SUNA) Revised: June 2020 Workgroup Members: AUA: Roxy Baumgartner, RN, APN-BC; Sam Chang, MD; Susan Flick, CNP; Howard Goldman, MD, FACS; Jim Kovarik, MS, PA-C; Yair Lotan, MD; Elspeth McDougall, MD, FRCSC, MHPE; Arthur Sagalowsky, MD; Edouard Trabulsi, MD SUNA: Debbie Hensley, RN; Christy Krieg, MSN, CUNP; Leanne Schimke, MSN, CUNP I. Statement of Purpose: To define the performance guidance surrounding the instillation of intravesical cytotoxic, immunotherapeutic, and/or therapeutic drugs via sterile technique catheterization for patients with non-muscle invasive bladder cancer (NMIBC, urothelial carcinoma). II. Population: Adult Urology III. Definition: Intravesical therapy involves instillation of a therapeutic agent directly into the bladder via insertion of a urethral catheter. IV. Indications: For administration of medication directly into the bladder via catheterization utilizing sterile technique for NMIBC treatment. V. Guidelines and Principles: Health care personnel (MD, NP, PA, RN, LPN, or MA) performing intravesical therapy must be educated, demonstrate competency, and understand the implications of non-muscle invasive bladder cancer. (Scope of practice for health care personnel listed may vary based on state or institution). This should include associated health and safety issues regarding handling of cytotoxic, and immunotherapeutic agents; and documented competency of safe practical skills. At a minimum, each institution or office practice setting should implement an established, annual competency program to review safety work practices and guidelines regarding storage, receiving, handling/ transportation, administration, disposal, and handling a spill of hazardous drugs. (Mellinger, 2010) VI. -
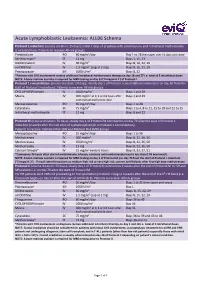
Acute Lymphoblastic Leukaemia: ALL06 Schema
Acute Lymphoblastic Leukaemia: ALL06 Schema Protocol 1 induction: (course duration: 35 days); initial 7 days of prephase with prednisolone and intrathecal methotrexate is included here. Patients to receive: All risk groups Prednisolone PO 60 mg/m2/day Day 1 to 28 then taper over 10 days and cease Methotrexate* IT 12 mg Days 1, 15, 33 DAUNOrubicin IV 30 mg/m2 Days 8, 16, 22, 29 vinCRISTine IV 1.5 mg/m2 (cap at 2 mg) Days 8, 16, 22, 29 Pegaspargase IM 1000 U/m2 Days 8, 22 *Patients with CNS involvement receive additional intrathecal methotrexate therapy on day 18 and 27 i.e. total of 5 intrathecal doses NOTE: A bone marrow aspirate is required for MRD testing on day 33 ('Timepoint 1') of Protocol I Protocol 1 consolidation: (course duration: 29 days); ideally day 1 of Protocol I consolidation commences on day 36 from the start of Protocol 1 induction ). Patients to receive: All risk groups. CYCLOPHOSPHamide IV 1000 mg/m2 Days 1 and 29 Mesna IV 400 mg/m2 at 0, 4 and 8 hours after Days 1 and 29 each cyclophosphamide dose Mercaptopurine PO 60 mg/m2/day Days 1 to 28 Cytarabine SC 75 mg/m2 Days 1 to 4, 8 to 11, 15 to 18 and 22 to 25 Intrathecal methotrexate IT 12 mg Days 8 and 22 Protocol M: (course duration: 56 days); ideally day 1 of Protocol M commences on day 79 after the start of Protocol 1 induction (2 weeks after the last dose of cyclophosphamide in Protocol 1 consolidation). -

Oncaspar, INN-Pegaspargase
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Oncaspar 750 U/ml solution for injection/infusion. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One ml of solution contains 750 Units (U)** of pegaspargase*. One vial of 5 ml solution contains 3,750 Units. * The active substance is a covalent conjugate of Escherichia coli-derived L-asparaginase with monomethoxypolyethylene glycol **One unit is defined as the quantity of enzyme required to liberate 1 µmol ammonia per minute at pH 7.3 and 37°C The potency of this medicinal product should not be compared to the one of another pegylated or non-pegylated protein of the same therapeutic class. For more information, see section 5.1. For the full list of excipients, see section 6.1. 3. PHARMACEUTICAL FORM Solution for injection/infusion. Clear, colourless solution. 4. CLINICAL PARTICULARS 4.1 Therapeutic indications Oncaspar is indicated as a component of antineoplastic combination therapy in acute lymphoblastic leukaemia (ALL) in paediatric patients from birth to 18 years, and adult patients. 4.2 Posology and method of administration Oncaspar should be prescribed and administered by physicians and/or health care personnel experienced in the use of antineoplastic products. It should only be given in a hospital setting where appropriate resuscitation equipment is available. Patients should be closely monitored for any adverse reactions throughout the administration period (see section 4.4). Posology Oncaspar is usually administered as part of combination chemotherapy protocols with other antineoplastic agents (see also section 4.5). Paediatric patients and adults ≤21 years The recommended dose in patients with a body surface area (BSA) ≥0.6 m2 and who are ≤21 years of age is 2,500 U of pegaspargase (equivalent to 3.3 ml Oncaspar)/m2 body surface area every 14 days. -

Cytostatics in Dutch Surface Water: Use, Presence and Risks To
Cytostatics in Dutch surface water Use, presence and risks to the aquatic environment RIVM Letter report 2018-0067 C. Moermond et al. Cytostatics in Dutch surface water Use, presence and risks to the aquatic environment RIVM Letter report 2018-0067 C. Moermond et al. RIVM Letter report 2018-0067 Colophon © RIVM 2018 Parts of this publication may be reproduced, provided acknowledgement is given to: National Institute for Public Health and the Environment, along with the title and year of publication. DOI 10.21945/RIVM-2018-0067 C. Moermond (auteur/coördinator), RIVM B. Venhuis (auteur/coördinator),RIVM M. van Elk (auteur), RIVM A. Oostlander (auteur), RIVM P. van Vlaardingen (auteur), RIVM M. Marinković (auteur), RIVM J. van Dijk (stagiair; auteur) RIVM Contact: Caroline Moermond VSP-MSP [email protected] This investigation has been performed by order and for the account of the Ministry of Infrastructure and Water management (IenW), within the framework of Green Deal Zorg en Ketenaanpak medicijnresten uit water. This is a publication of: National Institute for Public Health and the Environment P.O. Box 1 | 3720 BA Bilthoven The Netherlands www.rivm.nl/en Page 2 of 140 RIVM Letter report 2018-0067 Synopsis Cytostatics in Dutch surface water Cytostatics are important medicines to treat cancer patients. Via urine, cytostatic residues end up in waste water that is treated in waste water treatment plants and subsequently discharged into surface waters. Research from RIVM shows that for most cytostatics, their residues do not pose a risk to the environment. They are sufficiently metabolised in the human body and removed in waste water treatment plants. -

Asparaginase As Consolidation Therapy in Patients Undergoing Bone Marrow Transplantation for Acute Lymphoblastic Leukemia
Bone Marrow Transplantation, (1998) 21, 879–885 1998 Stockton Press All rights reserved 0268–3369/98 $12.00 http://www.stockton-press.co.uk/bmt Toxicity, pharmacology and feasibility of administration of PEG-L- asparaginase as consolidation therapy in patients undergoing bone marrow transplantation for acute lymphoblastic leukemia ML Graham1, BL Asselin2, JE Herndon II3, JR Casey1, S Chaffee1, GH Ciocci1, CW Daeschner4, AR Davis4, S Gold6, EC Halperin7, MJ Laughlin1, PL Martin8, JF Olson1 and J Kurtzberg1,9 Departments of 1Pediatrics, 3Community and Family Medicine, 5Pharmacy, 7Radiation Oncology and 9Pathology, Duke University Medical Center, Durham, NC; 2Department of Pediatrics, University of Rochester, Rochester, NY; 4Department of Pediatrics, Eastern Carolina University School of Medicine, Greenville; 6Department of Pediatrics, University of North Carolina School of Medicine, Chapel Hill; 8Department of Pediatrics, Bowman-Gray School of Medicine, Winston-Salem, NC, USA Summary: for bone marrow transplantation have occasionally been successful, but usually at the price of higher morbidity and We attempted to administer PEG-L-asparaginase (PEG- mortality rates, since most preparative regimens employ L-A) following hematologic recovery to 38 patients maximal or near maximal radiation and chemotherapy undergoing autologous or allogeneic marrow transplan- doses.6–8 tation for acute lymphoblastic leukemia (ALL). Twenty- Other strategies for reducing relapse have been explored. four patients (12 of 22 receiving allogeneic and 12 of 16 Since the development of graft-versus-host disease receiving autologous transplants) received between one (GVHD) in some series of allogeneic BMT has been asso- and 12 doses of PEG-L-A, including nine who com- ciated with a diminished relapse rate,9–12 Sullivan et al13 pleted the planned 12 doses of therapy.