Pseudobulbar Affect: When Patients Laugh Or Cry, but Don’T Know Why
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Primary Lateral Sclerosis, Upper Motor Neuron Dominant Amyotrophic Lateral Sclerosis, and Hereditary Spastic Paraplegia
brain sciences Review Upper Motor Neuron Disorders: Primary Lateral Sclerosis, Upper Motor Neuron Dominant Amyotrophic Lateral Sclerosis, and Hereditary Spastic Paraplegia Timothy Fullam and Jeffrey Statland * Department of Neurology, University of Kansas Medical Center, Kansas, KS 66160, USA; [email protected] * Correspondence: [email protected] Abstract: Following the exclusion of potentially reversible causes, the differential for those patients presenting with a predominant upper motor neuron syndrome includes primary lateral sclerosis (PLS), hereditary spastic paraplegia (HSP), or upper motor neuron dominant ALS (UMNdALS). Differentiation of these disorders in the early phases of disease remains challenging. While no single clinical or diagnostic tests is specific, there are several developing biomarkers and neuroimaging technologies which may help distinguish PLS from HSP and UMNdALS. Recent consensus diagnostic criteria and use of evolving technologies will allow more precise delineation of PLS from other upper motor neuron disorders and aid in the targeting of potentially disease-modifying therapeutics. Keywords: primary lateral sclerosis; amyotrophic lateral sclerosis; hereditary spastic paraplegia Citation: Fullam, T.; Statland, J. Upper Motor Neuron Disorders: Primary Lateral Sclerosis, Upper 1. Introduction Motor Neuron Dominant Jean-Martin Charcot (1825–1893) and Wilhelm Erb (1840–1921) are credited with first Amyotrophic Lateral Sclerosis, and describing a distinct clinical syndrome of upper motor neuron (UMN) tract degeneration in Hereditary Spastic Paraplegia. Brain isolation with symptoms including spasticity, hyperreflexia, and mild weakness [1,2]. Many Sci. 2021, 11, 611. https:// of the earliest described cases included cases of hereditary spastic paraplegia, amyotrophic doi.org/10.3390/brainsci11050611 lateral sclerosis, and underrecognized structural, infectious, or inflammatory etiologies for upper motor neuron dysfunction which have since become routinely diagnosed with the Academic Editors: P. -

Create a Healing Environment for Patients with Pseudobulbar Affect by Maude Mcgill, Phd, RN-BC, and Marzell Mcgill, BA, LPN
Strictly Clinical Create a healing environment for patients with pseudobulbar affect By Maude McGill, PhD, RN-BC, and Marzell McGill, BA, LPN tact their provider for prompt appropriate treatment. • Signs and symptoms include: Teach patients how to manage PBA • emotional responses (such as crying or laughing) for optimal quality of life. that don’t fit the situation • emotional episodes lasting longer than expected • sudden emotional outbursts (these outbursts can I just don’t understand why Mom is crying. When I include frustration, anger, laughter, or crying) walked in the room, she was happy and calm. Then, • moments of intense emotions that are out of the when I asked her about her day, her smile turned into patient’s control a frown and she started crying uncontrollably. I’ve nev - • facial expressions that don’t match the emotional er seen her cry like that. I’m concerned. response. EMOTIONS ARE NATURAL . We’ve all heard the cliché, “Emotions make us human.” People cry when they’re hurt and laugh when things are funny. However, mil - lions of people find that controlling their emotions is hard because of pseudobul - bar affect (PBA). PBA refers to inappropriate, involun - tary, or uncontrollable laughing or crying caused by the residual effects of a nervous system disorder. Al - though PBA is closely asso - ciated with post-stroke pa - tients, it can occur with any nervous system condition, including Alzheimer’s dis - ease, traumatic brain injury, and multiple sclerosis. Sci - entists have labeled PBA a disorder of emotional incon - tinence (patients can’t control their emotional respons - Navigating the new normal of PBA es). -

Management of Neurogenic Dysphagia
694 Postgrad Med J 2001;77:694–699 Postgrad Med J: first published as 10.1136/pmj.77.913.694 on 1 November 2001. Downloaded from Management of neurogenic dysphagia A M O Bakheit Dysphagia is common in patients with neuro- of the cerebral cortex, basal ganglia, brain logical disorders. It may result from lesions in stem, cerebellum, and lower cranial nerves may the central or peripheral nervous system as well result in dysphagia. Degeneration of the as from diseases of muscle and disorders of the myenteric ganglion cells in the oesophagus, neuromuscular junction. Drugs that are com- muscle diseases and disorders of neuromusc- monly used in the management of neurological ular transmission, for example myasthenia conditions may also precipitate or aggravate gravis and Eaton-Lambers syndrome, are other swallowing diYculties in some patients. Neuro- less common causes. genic dysphagia often results in serious compli- cations, including pulmonary aspiration, dehy- CEREBRAL CORTEX dration, and malnutrition. These The commonest condition associated with complications are usually preventable if the dysphagia resulting from cortical lesions is stroke. Acute stroke is complicated by dys- dysphagia is recognised early and managed 1 appropriately. phagia in about 25%–42% of all cases. Dysphagia in these patients is usually associ- Physiological mechanisms of neurogenic ated with hemiplegia due to lesions of the brain stem or the involvement of one or both dysphagia The act of swallowing may be viewed as three hemispheres. However, on rare occasions, dys- discrete but inter-related physiological stages: phagia may be the sole manifestation of a cer- the oral, pharyngeal, and oesophageal phases. -

IHH Notification Form Guide
Integrated Health Home Health Home Notification Form Guide Introduction This is to assist in providing the Health Home with information on criteria and how to enroll the member into an Integrated Health Home (IHH). Enrollment Criteria Member needs to 1. Be eligible for Medicaid. 2. Have 1 or more Serious Mental Illness (SMI) or Serious Emotional Disturbance (SED). 3. Meet the Functional Impairment (FI) as determined by a Licensed Mental Health Professional. SMI & SED Definitions Diagnosis must be determined by use the Diagnostic and Statistical Manual (DSM) of Mental Disorders published by the American Psychiatric Association or its most recent International Classification of Diseases (ICD). SMI is defined as an Adult that has a persistent or chronic mental illness, a behavioral, or emotional disorder that causes serious functional impairment and substantially interferes with or limits one or more major life activities including functioning in the family, school, employment or community. SMI may co-occur with substance use disorder, developmental, neurodevelopmental or intellectual disabilities but those diagnoses may not be the clinical focus for health home services. SED is defined by a Child having a diagnosable mental, behavioral or emotional disorder which results in a functional impairment that substantially interferes with or limits the child’s role or functioning in family, school, or community activities. SED may co-occur with substance use disorder, developmental, neurodevelopmental or intellectual disabilities but those diagnoses may not be the clinical focus for health home services. Mental Health Professional Definition Mental health professional meets all of the following conditions: 1. Holds at least a master’s degree in a mental health field including, but not limited to, psychology, counseling and guidance, psychiatric nursing and social work; or is a doctor of medicine or osteopathic medicine; and 2. -

Nuedexta, INN-Dextromethorphan/Quinidine
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT NUEDEXTA 15 mg/9 mg hard capsules 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each capsule contains dextromethorphan hydrobromide monohydrate, equivalent to 15.41 mg dextromethorphan and quinidine sulfate dihydrate, equivalent to 8.69 mg quinidine. Excipient with known effect: Each hard capsule contains 119.1 mg of lactose (as monohydrate). For the full list of excipients, see section 6.1. 3. PHARMACEUTICAL FORM Hard capsule Brick red gelatin capsule, size 1, with “DMQ / 20-10” printed in white ink on the capsule. 4. CLINICAL PARTICULARS 4.1 Therapeutic indications NUEDEXTA is indicated for the symptomatic treatment of pseudobulbar affect (PBA) in adults (see section 4.4). Efficacy has only been studied in patients with underlying Amyotrophic Lateral Sclerosis or Multiple Sclerosis (see section 5.1). 4.2 Posology and method of administration Posology The recommended starting dose is NUEDEXTA 15 mg/9 mg once daily. The recommended dose titration schedule is outlined below: Week 1 (day 1-7): The patient should take one NUEDEXTA 15 mg/9 mg capsule once daily, in the morning, for the initial 7 days. Weeks 2-4 (day 8-28): The patient should take one NUEDEXTA 15 mg/9 mg capsule, two times per day, one in the morning and one in the evening, 12-hours apart, for 21 days. From Week 4 on: If the clinical response with NUEDEXTA 15 mg/9 mg is adequate, the dose taken in weeks 2-4 should be continued. 2 If the clinical response with NUEDEXTA 15 mg/9 mg is inadequate, NUEDEXTA 23 mg/9 mg should be prescribed, taken two times per day, one in the morning and one in the evening, 12 hours apart. -

What's New in Psychopharmacology
Wednesday, 3:00 – 4:30, F2 What's New in Psychopharmacology Joel M. Sanchez, MD [email protected] Objectives: Identify advances in clinical assessment and management of selected healthcare issues related to persons with developmental disabilities Notes: 4/7/2016 What’s New in Nuedexta Psychopharmacology • A new medication for pseudobulbar affect (PBA) • Pseudobulbar Affect: Joel M. Sanchez, M.D. • Frequent, uncontrollable outbursts of crying or laughing in people with certain neurologic conditions or brain injuries Diplomate of the American Board of Psychiatry and Neurology, Psychiatry • Episodes may occur several times per day and can last seconds to minutes Diplomate of the American Board of Psychiatry and Neurology, Child/Adolescent Psychiatry • “emotional incontinence” • “pathological laughing and crying” The Right Door for Hope, Recovery and Wellness Wedgwood Christian Services Turning Leaf Behavioral Health Services Community Mental Health Authority - CEI ADHD Medications Antidepressants • Stimulants • SSRIs • Adzenys XR-ODT (amphetamine extended release oral dissolving tablet) • Brintellix (vortioxetine) • Quillivant XR (methylphenidate extended release liquid) • Viibryd (vilazodone) • Daytrana (methylphenidate transdermal) • SNRIs • Non-Stimulants • Fetzima (levomilnacipran) • Clonidine (Catapres / Kapvay) • Pristiq (desvenlafaxine) • Guanfacine (Tenex / Intuniv) • Strattera (atomoxetine) Antipsychotics Blood Pressure Medications • Abilify Maintena & Aristada (aripiprazole) • What’s Old is New • Every 4 weeks or Every 4-6 -

ICD9 & ICD10 Neuromuscular Codes
ICD-9-CM and ICD-10-CM NEUROMUSCULAR DIAGNOSIS CODES ICD-9-CM ICD-10-CM Focal Neuropathy Mononeuropathy G56.00 Carpal tunnel syndrome, unspecified Carpal tunnel syndrome 354.00 G56.00 upper limb Other lesions of median nerve, Other median nerve lesion 354.10 G56.10 unspecified upper limb Lesion of ulnar nerve, unspecified Lesion of ulnar nerve 354.20 G56.20 upper limb Lesion of radial nerve, unspecified Lesion of radial nerve 354.30 G56.30 upper limb Lesion of sciatic nerve, unspecified Sciatic nerve lesion (Piriformis syndrome) 355.00 G57.00 lower limb Meralgia paresthetica, unspecified Meralgia paresthetica 355.10 G57.10 lower limb Lesion of lateral popiteal nerve, Peroneal nerve (lesion of lateral popiteal nerve) 355.30 G57.30 unspecified lower limb Tarsal tunnel syndrome, unspecified Tarsal tunnel syndrome 355.50 G57.50 lower limb Plexus Brachial plexus lesion 353.00 Brachial plexus disorders G54.0 Brachial neuralgia (or radiculitis NOS) 723.40 Radiculopathy, cervical region M54.12 Radiculopathy, cervicothoracic region M54.13 Thoracic outlet syndrome (Thoracic root Thoracic root disorders, not elsewhere 353.00 G54.3 lesions, not elsewhere classified) classified Lumbosacral plexus lesion 353.10 Lumbosacral plexus disorders G54.1 Neuralgic amyotrophy 353.50 Neuralgic amyotrophy G54.5 Root Cervical radiculopathy (Intervertebral disc Cervical disc disorder with myelopathy, 722.71 M50.00 disorder with myelopathy, cervical region) unspecified cervical region Lumbosacral root lesions (Degeneration of Other intervertebral disc degeneration, -

History-Of-Movement-Disorders.Pdf
Comp. by: NJayamalathiProof0000876237 Date:20/11/08 Time:10:08:14 Stage:First Proof File Path://spiina1001z/Womat/Production/PRODENV/0000000001/0000011393/0000000016/ 0000876237.3D Proof by: QC by: ProjectAcronym:BS:FINGER Volume:02133 Handbook of Clinical Neurology, Vol. 95 (3rd series) History of Neurology S. Finger, F. Boller, K.L. Tyler, Editors # 2009 Elsevier B.V. All rights reserved Chapter 33 The history of movement disorders DOUGLAS J. LANSKA* Veterans Affairs Medical Center, Tomah, WI, USA, and University of Wisconsin School of Medicine and Public Health, Madison, WI, USA THE BASAL GANGLIA AND DISORDERS Eduard Hitzig (1838–1907) on the cerebral cortex of dogs OF MOVEMENT (Fritsch and Hitzig, 1870/1960), British physiologist Distinction between cortex, white matter, David Ferrier’s (1843–1928) stimulation and ablation and subcortical nuclei experiments on rabbits, cats, dogs and primates begun in 1873 (Ferrier, 1876), and Jackson’s careful clinical The distinction between cortex, white matter, and sub- and clinical-pathologic studies in people (late 1860s cortical nuclei was appreciated by Andreas Vesalius and early 1870s) that the role of the motor cortex was (1514–1564) and Francisco Piccolomini (1520–1604) in appreciated, so that by 1876 Jackson could consider the the 16th century (Vesalius, 1542; Piccolomini, 1630; “motor centers in Hitzig and Ferrier’s region ...higher Goetz et al., 2001a), and a century later British physician in degree of evolution that the corpus striatum” Thomas Willis (1621–1675) implicated the corpus -

Deep Brain Stimulation in the Treatment of Dyskinesia and Dystonia
Neurosurg Focus 17 (1):E2, 2004, Click here to return to Table of Contents Deep brain stimulation in the treatment of dyskinesia and dystonia HIROKI TODA, M.D., PH.D., CLEMENT HAMANI, M.D., PH.D., AND ANDRES LOZANO, M.D., PH.D., F.R.C.S.(C) Division of Neurosurgery, Department of Surgery, Toronto Western Hospital, University of Toronto, Ontario, Cananda Deep brain stimulation (DBS) has become a mainstay of treatment for patients with movement disorders. This modality is directed at modulating pathological activity within basal ganglia output structures by stimulating some of their nuclei, such as the subthalamic nucleus (STN) and the globus pallidus internus (GPi), without making permanent lesions. With the accumulation of experience, indications for the use of DBS have become clearer and the effective- ness and limitations of this form of therapy in different clinical conditions have been better appreciated. In this review the authors discuss the efficacy of DBS in the treatment of dystonia and levodopa-induced dyskinesias. The use of DBS of the STN and GPi is very effective for the treatment of movement disorders induced by levodopa. The relative ben- efits of using the GPi as opposed to the STN as a target are still being investigated. Bilateral GPi stimulation is gain- ing importance in the therapeutic armamentarium for the treatment of dystonia. The DYT1 forms of generalized dys- tonia and cervical dystonias respond to DBS better than secondary dystonia does. Discrimination between the diverse forms of dystonia and a better understanding of the pathophysiological features of this condition will serve as a plat- form for improved outcomes. -

THE SYNDROMES of the ARTERIES of the BRAIN and SPINAL CORD Part 1 by LESLIE G
65 Postgrad Med J: first published as 10.1136/pgmj.29.328.65 on 1 February 1953. Downloaded from THE SYNDROMES OF THE ARTERIES OF THE BRAIN AND SPINAL CORD Part 1 By LESLIE G. KILOH, M.D., M.R.C.P., D.P.M. First Assistant in the Joint Department of Psychological Medicine, Royal Victoria Infirmary and University of Durham Introduction general circulatory efficiency at the time of the Little interest is shown at present in the syn- catastrophe is of the greatest importance. An dromes of the arteries of the brain and spinal cord, occlusion, producing little effect in the presence of and this perhaps is related to the tendency to a normal blood pressure, may cause widespread minimize the importance of cerebral localization. pathological changes if hypotension co-exists. Yet these syndromes are far from being fully A marked discrepancy has been noted between elucidated and understood. It must be admitted the effects of ligation and of spontaneous throm- that in many cases precise localization is often of bosis of an artery. The former seldom produces little more than academic interest and ill effects whilst the latter frequently determines provides Protected by copyright. nothing but a measure of personal satisfaction to infarction. This is probably due to the tendency the physician. But it is worth recalling that the of a spontaneous thrombus to extend along the detailed study of the distribution of the bronchi affected vessel, sealing its branches and blocking and of the pathological anatomy of congenital its collateral circulation, and to the fact that the abnormalities of the heart, was similarly neglected arterial disease is so often generalized. -

Part Ii – Neurological Disorders
Part ii – Neurological Disorders CHAPTER 14 MOVEMENT DISORDERS AND MOTOR NEURONE DISEASE Dr William P. Howlett 2012 Kilimanjaro Christian Medical Centre, Moshi, Kilimanjaro, Tanzania BRIC 2012 University of Bergen PO Box 7800 NO-5020 Bergen Norway NEUROLOGY IN AFRICA William Howlett Illustrations: Ellinor Moldeklev Hoff, Department of Photos and Drawings, UiB Cover: Tor Vegard Tobiassen Layout: Christian Bakke, Division of Communication, University of Bergen E JØM RKE IL T M 2 Printed by Bodoni, Bergen, Norway 4 9 1 9 6 Trykksak Copyright © 2012 William Howlett NEUROLOGY IN AFRICA is freely available to download at Bergen Open Research Archive (https://bora.uib.no) www.uib.no/cih/en/resources/neurology-in-africa ISBN 978-82-7453-085-0 Notice/Disclaimer This publication is intended to give accurate information with regard to the subject matter covered. However medical knowledge is constantly changing and information may alter. It is the responsibility of the practitioner to determine the best treatment for the patient and readers are therefore obliged to check and verify information contained within the book. This recommendation is most important with regard to drugs used, their dose, route and duration of administration, indications and contraindications and side effects. The author and the publisher waive any and all liability for damages, injury or death to persons or property incurred, directly or indirectly by this publication. CONTENTS MOVEMENT DISORDERS AND MOTOR NEURONE DISEASE 329 PARKINSON’S DISEASE (PD) � � � � � � � � � � � -
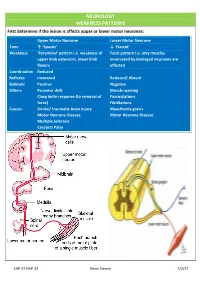
NEUROLOGY WEAKNESS PATTERNS First Determine If the Lesion Is Affects Upper Or Lower Motor Neurones
NEUROLOGY WEAKNESS PATTERNS First determine if the lesion is affects upper or lower motor neurones: Upper Motor Neurone Lower Motor Neurone Tone ↑ ‘Spastic’ ↓ ‘Flaccid’ Weakness ‘Pyramidal’ pattern i.e. weakness of Focal pattern i.e. only muscles upper limb extensors, lower limb innervated by damaged neurones are flexors affected Coordination Reduced Reflexes Increased Reduced/ Absent Babinski Positive Negative Others Pronator drift Muscle wasting Clasp knife response (to removal of Fasciculations force) Fibrillations Causes Stroke/ traumatic brain injury Myasthenia gravis Motor Neurone Disease Motor Neurone Disease Multiple Sclerosis Cerebral Palsy CAP 37 HAP 33 Kevin Gervin 7/2/17 What do we mean by extrapyramidal? Extrapyramidal symptoms Symptoms affecting tracts other than corticospinal and corticobulbar Extrapyramidal tracts don’t travel through the medullary pyramids The system regulates posture and muscle tone so pathology usually leads to movement disorders Most common cause is typical antipsychotics affecting Dopamine (D2) receptors e.g. haloperidol Treatment is anticholinergics e.g. procyclidine Extrapyramidal conditions: Acute dystonic reactions → muscle spasms e.g. neck, jaw, back. Akathisia- feeling of internal restlessness Drug induced Parkinsonism- tremor, rigidity etc. Tardive dyskinesia- involuntary muscle movements of lower face and extremities, often permanent What’s the difference between a Bulbar and Pseudobulbar palsy? Bulbar Pseudobulbar Pathophysiology LMN lesion CN V, VII, IX- XII Disease of corticobulbar