Microtubule-Associated Protein-4 Controls Nanovesicle Dynamics And
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Regulation of Cardiovascular Homeostasis by Autophagy
Georgia State University ScholarWorks @ Georgia State University Chemistry Dissertations Department of Chemistry 12-16-2020 Regulation Of Cardiovascular Homeostasis By Autophagy Jing Mu Georgia State University Follow this and additional works at: https://scholarworks.gsu.edu/chemistry_diss Recommended Citation Mu, Jing, "Regulation Of Cardiovascular Homeostasis By Autophagy." Dissertation, Georgia State University, 2020. https://scholarworks.gsu.edu/chemistry_diss/190 This Dissertation is brought to you for free and open access by the Department of Chemistry at ScholarWorks @ Georgia State University. It has been accepted for inclusion in Chemistry Dissertations by an authorized administrator of ScholarWorks @ Georgia State University. For more information, please contact [email protected]. REGULATION OF CARDIOVASCULAR HOMEOSTASIS BY AUTOPHAGY by JING MU Under the Direction of Ming-hui Zou, MD/PhD ABSTRACT Macroautophagy (hereafter autophagy) is a fundamental cellular process that removes unnecessary or dysfunctional components. It allows the orderly degradation and recycling of cellular components. Mitophagy refers to the selective removal of damaged mitochondria via autophagy pathway. In addition to utilizing core autophagic machinery components, mitophagy exploits a variety of molecules, such as PTEN-induced putative kinase protein 1 (PINK1) and Parkin, to identify and eliminate damaged or superfluous mitochondria. Dysregulation of autophagy and mitophagy contributes to a variety of human disorders, including cardiovascular diseases, such as atherosclerosis and diabetic cardiomyopathy. Vascular smooth muscle cells (VSMCs) are a major component of the vascular media, and are vital for maintaining vessel homeostasis. Migration of VSMCs from the media to intima occurs during the development of atherosclerosis. Although alterations in autophagy activity have been reported in atherosclerosis, further investigation is required to delineate the mechanism by which autophagy regulates microtubule stability and cell migration. -

Dissertation Kokes M
Mechanisms of Chlamydia Manipulation of Host Cell Biology Revealed Through Genetic Approaches by Marcela Kokes Department of Molecular Genetics and Microbiology Program in Cell and Molecular Biology Duke University Date:_______________________ Approved: ___________________________ Raphael H. Valdivia, Supervisor ___________________________ Daniel J. Lew ___________________________ Jörn Coers ___________________________ Patrick C. Seed Dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the Department of Molecular Genetics and Microbiology in the Graduate School of Duke University 2015 i v ABSTRACT Mechanisms of Chlamydia Manipulation of Host Cell Biology Revealed Through Genetic Approaches by Marcela Kokes Department of Molecular Genetics and Microbiology Program in Cell and Molecular Biology Duke University Date:_______________________ Approved: ___________________________ Raphael H. Valdivia, Supervisor ___________________________ Daniel J. Lew ___________________________ Jörn Coers ___________________________ Patrick C. Seed An abstract of a dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the Department of Molecular Genetics and Microbiology in the Graduate School of Duke University 2015 Copyright by Marcela Kokes 2015 i v Abstract Chlamydia trachomatis is the most common sexually transmitted bacterial pathogen and is the leading cause of preventable blindness worldwide. Chlamydia is particularly intriguing from the perspective of cell biology because it is an obligate intracellular pathogen that manipulates host cellular pathways to ensure its proliferation and survival. This is achieved through a significant remodeling of the host cell’s internal architecture from within a membrane-bound vacuole, termed the inclusion. However, given a previous lack of tools to perform genetic analysis, the mechanisms by which Chlamydia induces host cellular changes remained unclear. -

Chromatography Including Electrophoresis and Other Separation Methods
15SIO 06£ i 36 j 6 VOL. 610 NO.2 JUNE 12, 1992 THIS ISSUE COMPLETES VOL. 610 Bibliography Section JOURNAL OF CHROMATOGRAPHY INCLUDING ELECTROPHORESIS AND OTHER SEPARATION METHODS EDITORS U. A. Th. Brinkman (Amsterdam) R. W. Giese (Boston, MA) J. K. Haken (Kensington, N.S.W.) K. Macek (Prague) L. R. Snyder (Orinda, CA) EDITORS. SYMPOSIUM VOLUMES. E. Heftmann (Orinda. CAl. Z. Deyl (Prague) EDITORIAL BOARD D. W. Armstrong (Rollo. MO) W. A. Aue (Holifo,) P. Botek (8.00) A. A. Boulton (Saskatoon) P. W. Cmr (Minneopolis. MN) N. H. C. Cooke (San Ramon. CAl V. A. Davankov (Moscow) Z. Deyl (Progue) S. Dilli (Kl~nsington. N.S.W.) F. Ern! (Basle) M. B. Evans (Hatfield) J. L. Glojcl, (N. Billerico. MA) G. A. Guiochon (Knoxville, TN) P. R. Haddod (Kensington. N.S.W.) I. M. Hais (Hradec Kralove) W. S. Hancock (San FranCISCo. CAl S. Hjerten (Uppsalo) Cs. Horvinh (New Haven. CT) J. F. K. Huber (Vienna) K.·P. Hupe (Woldbronn) T. W. Hutchens (Houston. IX) J. Jonak (B.oo) P. Jandera (Pardubice) B. L. Kmger (B05<on. MA) J. J. Kirkland (Wilmington. DE) E. sz. Kovats (Lausanne) A. J. P. Mortin (Cambridge) L. W. McLoughlin (Chestnut Hill. MA) E. D. Morgan (Keele) J. O. Pearson (KrJlamazoo, MI) H. Poppe (Amsterdam) F. E. Regnier (West Lafayette. IN) P. G. Righetti (Milan) P. Sclloenmakers (Eindhoven) R. Schwarzenbach (Dubendor!) R. E. Shoup (West Lafayette. IN) A. M. Siouf!i (Mo,seille) D. J. Strydom (Boston. MA) N. Tonaka (Kyoto) S. Terabe (Hyogo) K. K. Unger (Mainz) R. Verpoorle (Leiden) Gy. -

Rho Guanosine Triphosphatase Mediates the Selective Stabilization of Microtubules Induced by Lysophosphatidic Acid Tiffani A
Rho Guanosine Triphosphatase Mediates the Selective Stabilization of Microtubules Induced by Lysophosphatidic Acid Tiffani A. Cook,*‡ Takayuki Nagasaki,* and Gregg G. Gundersen*i *Department of Anatomy and Cell Biology, ‡Integrated Program in Cellular, Molecular, and Biophysical Studies, and iDepartment of Pathology, Columbia University, New York Abstract. The asymmetric distribution of stable, post- MTs and, in wound-edge cells, these stable MTs were translationally modified microtubules (MTs) contrib- appropriately oriented toward the leading edge of the utes to the polarization of many cell types, yet the fac- cell. LPA had little effect on individual parameters of tors controlling the formation of these MTs are not MT dynamics, but did induce long states of pause in a known. We have found that lysophosphatidic acid subset of MTs near the edge of the cell. Rho stimula- (LPA) is a major serum factor responsible for rapidly tion of MT stability was independent of actin stress fi- Downloaded from generating stable, detyrosinated (Glu) MTs in serum- ber formation. These results identify rho as a novel reg- starved 3T3 cells. Using C3 toxin and val14 rho we ulator of the MT cytoskeleton that selectively stabilizes showed that rho was both necessary and sufficient for MTs during cell polarization by acting as a switch be- the induction of Glu MTs by LPA and serum. Unlike tween dynamic and stable states of MTs rather than as previously described factors that induce MT stability, a modulator of MT assembly and disassembly. rho induced the stabilization of only a subset of the jcb.rupress.org n many cells there are at least two populations of mi- et al., 1984, 1987; Gundersen and Bulinski, 1986; Webster crotubules (MTs)1 distinguishable by their rates of et al., 1987; Kreis, 1987) and/or acetylated tubulin (Pip- on November 19, 2017 I turnover. -

Microtubule Acetylation but Not Detyrosination Promotes Focal
© 2019. Published by The Company of Biologists Ltd | Journal of Cell Science (2019) 132, jcs225805. doi:10.1242/jcs.225805 SHORT REPORT Microtubule acetylation but not detyrosination promotes focal adhesion dynamics and astrocyte migration Bertille Bance1,2,*, Shailaja Seetharaman1,3,*, Cécile Leduc1, Batiste Boëda1 and Sandrine Etienne-Manneville1,‡ ABSTRACT Kaverina et al., 2002; Palazzo et al., 2004; Ezratty et al., 2009; Microtubules play a crucial role in mesenchymal migration by Stehbens et al., 2014). The polarized regulation of integrin-based controlling cell polarity and the turnover of cell adhesive structures on structures implies that the functions of microtubules must be the extracellular matrix. The polarized functions of microtubules imply controlled in a polarized manner. that microtubules are locally regulated. Here, we investigated the Several mechanisms, including MAP interactions and post- α β regulation and role of two major tubulin post-translational modifications, translational modifications of -and -tubulin (Janke, 2014; Song acetylation and detyrosination, which have been associated with stable and Brady, 2015; Etienne-Manneville, 2010; Strzyz, 2016; Aillaud microtubules. Using primary astrocytes in a wound healing assay, we et al., 2016), affect microtubule dynamics or microtubule association show that these tubulin modifications are independently regulated with protein partners (Janke, 2014; Raunser and Gatsogiannis, 2015; α during cell polarization and differently affect cell migration. In contrast to Yu et al., 2015; Song and Brady, 2015). TAT1 [also known as microtubule detyrosination, αTAT1 (ATAT1)-mediated microtubule ATAT1, and as Mec-17 in Caenorhabditis elegans (Akella et al., acetylation increases in the vicinity of focal adhesions and promotes 2010)] is the major tubulin acetyltransferase in mammals. -

The Tubulin Code and Its Role in Controlling Microtubule Properties and Functions
REVIEWS The tubulin code and its role in controlling microtubule properties and functions Carsten Janke 1,2 ✉ and Maria M. Magiera 1,2 ✉ Abstract | Microtubules are core components of the eukaryotic cytoskeleton with essential roles in cell division, shaping, motility and intracellular transport. Despite their functional heterogeneity , microtubules have a highly conserved structure made from almost identical molecular building blocks: the tubulin proteins. Alternative tubulin isotypes and a variety of post- translational modifications control the properties and functions of the microtubule cytoskeleton, a concept known as the ‘tubulin code’. Here we review the current understanding of the molecular components of the tubulin code and how they impact microtubule properties and functions. We discuss how tubulin isotypes and post-translational modifications control microtubule behaviour at the molecular level and how this translates into physiological functions at the cellular and organism levels. We then go on to show how fine-tuning of microtubule function by some tubulin modifications can affect homeostasis and how perturbation of this fine- tuning can lead to a range of dysfunctions, many of which are linked to human disease. 20 Axonemes Microtubules are cytoskeletal filaments with an outer cellular structures . Other MAPs bind to the micro- A tubular structure built from diameter of approximately 25 nm, and are composed of tubule lattice along its entire length and are thus con- microtubules and associated heterodimers of globular α- tubulin and β- tubulin mol- sidered to regulate microtubule dynamics and stability, proteins at the core of all ecules. As they are hollow cylinders, microtubules are but might also have additional roles that in many cases eukaryotic cilia and flagella. -

Cap-Gly Proteins at Microtubule Plus Ends: Is EB1 Detyrosination Involved?
Cap-Gly Proteins at Microtubule Plus Ends: Is EB1 Detyrosination Involved? Anouk Bosson, Jean-Marc Soleilhac, Odile Valiron, Didier Job, Annie Andrieux, Marie-Jo Moutin* Institut National de la Sante´ et de la Recherche Me´dicale (INSERM) U836, Grenoble Institut des Neurosciences, Commissariat a` l’Energie Atomique (CEA) Institut de Recherches en Technologies et Sciences pour le Vivant - Groupe Physiopathologie du Cytosquelette (iRTSV-GPC), Universite´ Joseph Fourier, Grenoble, France Abstract Localization of CAP-Gly proteins such as CLIP170 at microtubule+ends results from their dual interaction with a-tubulin and EB1 through their C-terminal amino acids 2EEY. Detyrosination (cleavage of the terminal tyrosine) of a-tubulin by tubulin- carboxypeptidase abolishes CLIP170 binding. Can detyrosination affect EB1 and thus regulate the presence of CLIP170 at microtubule+ends as well? We developed specific antibodies to discriminate tyrosinated vs detyrosinated forms of EB1 and detected only tyrosinated EB1 in fibroblasts, astrocytes, and total brain tissue. Over-expressed EB1 was not detyrosinated in cells and chimeric EB1 with the eight C-terminal amino acids of a-tubulin was only barely detyrosinated. Our results indicate that detyrosination regulates CLIPs interaction with a-tubulin, but not with EB1. They highlight the specificity of carboxypeptidase toward tubulin. Citation: Bosson A, Soleilhac J-M, Valiron O, Job D, Andrieux A, et al. (2012) Cap-Gly Proteins at Microtubule Plus Ends: Is EB1 Detyrosination Involved? PLoS ONE 7(3): e33490. doi:10.1371/journal.pone.0033490 Editor: Thierry Soldati, Universite´ de Gene`ve, Switzerland Received November 18, 2011; Accepted February 15, 2012; Published March 14, 2012 Copyright: ß 2012 Bosson et al. -

Microtubule Tyrosination/Detyrosination Specifies a Mitotic Error Code
bioRxiv preprint doi: https://doi.org/10.1101/801977; this version posted October 11, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Microtubule tyrosination/detyrosination specifies a mitotic error code Luísa T. Ferreira1,2, Bernardo Orr1,2, Girish Rajendraprasad3, António J. Pereira1,2, Carolina Lemos2,4,5, Joana T. Lima1,2, Clàudia Guasch Boldú3, Jorge G. Ferreira1,2,6, Marin Barisic3,7, Helder Maiato1,2,6* 1 Chromosome Instability & Dynamics Group, i3S - Instituto de Investigação e Inovação em Saúde, Universidade do Porto, Rua Alfredo Allen 208, 4200-135 Porto, Portugal 2 Instituto de Biologia Molecular e Celular, Universidade do Porto, Rua Alfredo Allen 208, 4200-135 Porto, Portugal 3 Cell Division and Cytoskeleton, Danish Cancer Society Research Center (DCRC), Strandboulevarden 49, 2100 Copenhagen, Denmark 4 UnIGENe, i3S - Instituto de Investigação e Inovação em Saúde, Universidade do Porto, Rua Alfredo Allen 208, 4200-135 Porto, Portugal 5 Instituto de Ciências Biomédicas Abel Salazar, Universidade do Porto, Rua Jorge Viterbo Ferreira, 228, 4050-313 Porto, Portugal 6 Cell Division Group, Experimental Biology Unit, Department of Biomedicine, Faculdade de Medicina, Universidade do Porto, Alameda Prof. Hernâni Monteiro, 4200-319 Porto, Portugal 7 Department of Cellular and Molecular Medicine, Faculty of Health Sciences, University of Copenhagen, Blegdamsvej 3B, 2200 Copenhagen, Denmark *Correspondence to: [email protected] 1 bioRxiv preprint doi: https://doi.org/10.1101/801977; this version posted October 11, 2019. -

Post-Translational Modifications Regulate Microtubule Function
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by MPG.PuRe REVIEWS POST-TRANSLATIONAL MODIFICATIONS REGULATE MICROTUBULE FUNCTION Stefan Westermann* and Klaus Weber ‡ The αβ-tubulin heterodimer, the building block of microtubules, is subject to a large number of post-translational modifications, comparable in diversity to the intensively studied histone modifications. Although these unusual modifications are conserved throughout evolution, their functions have remained almost completely elusive. Recently, however, important advances in the understanding of how tubulin modifications regulate function and organization have been made. αβ MITOTIC SPINDLE Microtubules are an important component of the domain of -tubulins, which is located on the outside 3 A bipolar array of microtubules cytoskeleton and carry out a variety of essential func- of the microtubule where it is well positioned to influ- that functions to move the tions. During cell division, microtubules form the ence interactions with other proteins. duplicated chromosomes MITOTIC SPINDLE, the structure that is required to faith- Here,we review the literature on the occurrence of during mitosis and meiosis. fully segregate replicated sister chromatids. Together these modifications, the enzymes that are involved in AXONEME with accessory proteins, they constitute the AXONEME of generating them, and their potential functions. A bundle of microtubules and CILIA and FLAGELLA and so contribute to cell motility. In associated proteins that form the addition, they are important factors in the generation The role of the tubulin tails core of a flagellum or cilium. of cell polarity and also function as tracks along which Electron crystallographic studies of the αβ-tubulin CILIA — with the help of motor proteins — organelles and dimer have provided important insights into its struc- Hair-like extensions of cells, vesicles are transported through the cell. -

Subpellicular and Flagellar Microtubules of Trypanosoma
Journal of Cell Science 110, 431-437 (1997) 431 Printed in Great Britain © The Company of Biologists Limited 1997 JCS3502 Subpellicular and flagellar microtubules of Trypanosoma brucei are extensively glutamylated André Schneider1, Uwe Plessmann2 and Klaus Weber2,* 1University of Fribourg, Institute of Zoology, Pérolles, CH 1700 Fribourg, Switzerland 2Max Planck Institute for Biophysical Chemistry, Department of Biochemistry, PO Box 2841, D-37018, Goettingen, Germany *Author for correspondence SUMMARY To determine the spectrum of tubulin variants in cytoskel- residues 445 of α- and 435 of β-tubulin. The same sites are etons of Trypanosoma brucei carboxy-terminal fragments used in glutamylated tubulins of mammalian brain. No of α- and β-tubulin were isolated and characterized by tubulin variants based on polyglycylation are detected in sequencing and mass spectrometry. All variants arise by cytoskeletal preparations or in isolated flagella. Tubulin posttranslational modifications. We confirm the presence of specific incorporation of radioactive glutamate but not of tyrosinated and detyrosinated α-tubulin. Unexpectedly, but glycine is observed when protein biosynthesis is completely in line with its sequence, β-tubulin also occurs with and inhibited in Trypanosoma cells. Possible reasons for the without its carboxy-terminal tyrosine. Both tyrosinated absence of polyglycylated tubulins from the trypanosomal and detyrosinated α- and β-tubulins are extensively glu- axoneme are discussed. Finally we show that lysine 40 of tamylated. Unglutamylated tubulins are only trace compo- the flagellar α-tubulin is completely acetylated. nents of the cytoskeletal microtubules. The maximal numbers of glutamyl residues in the lateral chain are 15 and 6 for α- and β-tubulin, respectively. The oligoglutamyl Key words: Acetylation, Carboxypeptidase, Polyglutamylation, side chain is linked via an isopeptide bond to glutamic acid Posttranslational modification, Tyrosination INTRODUCTION and β-tubulin. -

Microtubules Provide a Viscoelastic Resistance to Myocyte Motion
bioRxiv preprint doi: https://doi.org/10.1101/361824; this version posted July 3, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Microtubules provide a viscoelastic resistance to myocyte motion. M. A. Caporizzo, Ph.D.,1 C. Y. Chen, Ph.D.,1 A. K. Salomon,1 K. Bedi, Ph.D.,2 K. B. Margulies, M.D.,1, 2 B. L. Prosser, Ph.D.1 Working Title: Microtubules dampen cardiomyocyte motion Manuscript Word Count excluding references and figure legends: 5244 Subject Terms: Basic Science Research, Contractile Function, Mechanisms 1 | P a g e bioRxiv preprint doi: https://doi.org/10.1101/361824; this version posted July 3, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. ABSTRACT Background: Microtubules (MT) buckle and bear load during myocyte contraction, a behavior enhanced by post-translational detyrosination. This buckling suggests a spring-like resistance against myocyte shortening, which could store energy and aid myocyte relaxation. Despite this visual suggesting of elastic behavior, the precise mechanical contribution of the cardiac MT network remains to be defined. Methods: Here we experimentally and computationally probe the mechanical contribution of stable microtubules and their influence on myocyte function. We use multiple approaches to interrogate viscoelasticity and cell shortening in primary murine myocytes where either MTs are depolymerized or detyrosination is suppressed, and use the results to inform a mathematical model of myocyte viscoelasticity. -
View PDF Version
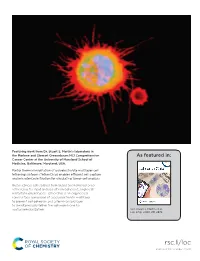
Featuring work from Dr. Stuart S. Martin’s laboratory in the Marlene and Stewart Greenebaum NCI Comprehensive As featured in: Cancer Center at the University of Maryland School of Medicine, Baltimore, Maryland, USA. Partial thermal imidization of polyelectrolyte multilayer cell tethering surfaces (TetherChip) enables effi cient cell capture and microtentacle fi xation for circulating tumor cell analysis Breast cancer cell isolated from blood and tethered onto TetherChip for rapid analysis of non-adherent, single-cell metastatic phenotypes. TetherChip is an engineered nanosurface comprised of a polyelectrolyte multilayer to prevent cell adhesion and a terminal lipid layer to simultaneously tether the cell membrane for spatial immobilization. See Stuart S. Martin et al., Lab Chip, 2020, 20, 2872. rsc.li/loc Registered charity number: 207890 Lab on a Chip View Article Online PAPER View Journal | View Issue Partial thermal imidization of polyelectrolyte Cite this: Lab Chip,2020,20,2872 multilayer cell tethering surfaces (TetherChip) enables efficient cell capture and microtentacle fixation for circulating tumor cell analysis† Julia A. Ju, ‡a Cornell J. Lee,‡a Keyata N. Thompson,a Eleanor C. Ory,a Rachel M. Lee, a Trevor J. Mathias, ab Stephen J. P. Pratt,ac Michele I. Vitolo, abd Christopher M. Jewell ef and Stuart S. Martin*abdf The technical challenges of imaging non-adherent tumor cells pose a critical barrier to understanding tumor cell responses to the non-adherent microenvironments of metastasis, like the bloodstream or lymphatics. In this study, we optimized a microfluidic device (TetherChip) engineered to prevent cell adhesion with an optically-clear, thermal-crosslinked polyelectrolyte multilayer nanosurface and a terminal Creative Commons Attribution 3.0 Unported Licence.