1 General Introduction
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Gymnorhina Tibicen Global Invasive
FULL ACCOUNT FOR: Gymnorhina tibicen Gymnorhina tibicen System: Terrestrial Kingdom Phylum Class Order Family Animalia Chordata Aves Passeriformes Cracticidae Common name Synonym Coracias tibicen Similar species Grallina cyanoleuca, Cracticus nigrogularis Summary Gymnorhina tibicen (the Australian magpie) was originally introduced to New Zealand around the 1860s in an attempt to combat pastoral pests. It is known to be extremely territorial, especially during the breeding season, and is known to assault other avian species and even humans. Magpies potentially threaten a number of indigenous avian fauna, as well as putting humans at risk of injury. view this species on IUCN Red List Species Description The Australian magpie (Gymnorhina tibicen), is a medium-sized ground-feeding passerine found throughout much of the Australian continent. They range from 36-44cm in length and weigh 280-340g with black and white plumage, black heads, wings and underparts together with white napes and shoulders (NRC, 1998). The iris of the adult magpie is red, whereas the juveniles' eyes are dark brown in colour. Minor differences exist between the male and female magpies, though in general, magpies are not considered to be sexually dimorphic (Simpson et al., 1993). Notes Although Australian magpies (Gymnorhina tibicen) have been known to have detrimental impacts on some birds, they can actually be beneficial to others. They do this by attacking and displacing common avian predators, such as harrier hawks and ravens, which in turn provides safe nesting grounds for a number of rural birds (Morgan et al, 2005). Lifecycle Stages The average life span of the Australian magpie (Gymnorhina tibicen) has not been studied in detail, but is estimated to be around 24 years, with some individuals living up to 30 years of age (Reilly, 1988). -

Bontebok Birds
Birds recorded in the Bontebok National Park 8 Little Grebe 446 European Roller 55 White-breasted Cormorant 451 African Hoopoe 58 Reed Cormorant 465 Acacia Pied Barbet 60 African Darter 469 Red-fronted Tinkerbird * 62 Grey Heron 474 Greater Honeyguide 63 Black-headed Heron 476 Lesser Honeyguide 65 Purple Heron 480 Ground Woodpecker 66 Great Egret 486 Cardinal Woodpecker 68 Yellow-billed Egret 488 Olive Woodpecker 71 Cattle Egret 494 Rufous-naped Lark * 81 Hamerkop 495 Cape Clapper Lark 83 White Stork n/a Agulhas Longbilled Lark 84 Black Stork 502 Karoo Lark 91 African Sacred Ibis 504 Red Lark * 94 Hadeda Ibis 506 Spike-heeled Lark 95 African Spoonbill 507 Red-capped Lark 102 Egyptian Goose 512 Thick-billed Lark 103 South African Shelduck 518 Barn Swallow 104 Yellow-billed Duck 520 White-throated Swallow 105 African Black Duck 523 Pearl-breasted Swallow 106 Cape Teal 526 Greater Striped Swallow 108 Red-billed Teal 529 Rock Martin 112 Cape Shoveler 530 Common House-Martin 113 Southern Pochard 533 Brown-throated Martin 116 Spur-winged Goose 534 Banded Martin 118 Secretarybird 536 Black Sawwing 122 Cape Vulture 541 Fork-tailed Drongo 126 Black (Yellow-billed) Kite 547 Cape Crow 127 Black-shouldered Kite 548 Pied Crow 131 Verreauxs' Eagle 550 White-necked Raven 136 Booted Eagle 551 Grey Tit 140 Martial Eagle 557 Cape Penduline-Tit 148 African Fish-Eagle 566 Cape Bulbul 149 Steppe Buzzard 572 Sombre Greenbul 152 Jackal Buzzard 577 Olive Thrush 155 Rufous-chested Sparrowhawk 582 Sentinel Rock-Thrush 158 Black Sparrowhawk 587 Capped Wheatear -

Cfreptiles & Amphibians
WWW.IRCF.ORG/REPTILESANDAMPHIBIANSJOURNALTABLE OF CONTENTS IRCF REPTILES & AMPHIBIANS IRCF REPTILES • VOL15, &NO AMPHIBIANS 4 • DEC 2008 189 • 23(1):51–61 • APR 2016 IRCF REPTILES & AMPHIBIANS CONSERVATION AND NATURAL HISTORY TABLE OF CONTENTS TRAVELOGUE FEATURE ARTICLES . Chasing Bullsnakes (Pituophis catenifer sayi) in Wisconsin: TexasOn the Road to Understanding Tech the Ecology and University Conservation of the Midwest’s Giant Serpent Students ...................... Joshua M. Kapfer 190 . The Shared History of Treeboas (Corallus grenadensis) and Humans on Grenada: A Hypothetical Excursion ............................................................................................................................Robert W. Henderson 198 RESEARCH ARTICLESTake On Zimbabwe . The Texas Horned Lizard in Central and Western Texas ....................... Emily Henry, Jason Brewer, Krista Mougey, and Gad Perry 204 . The Knight Anole (Anolis equestrisKaitlin) in Florida Danis, Ashley Hogan, and Kirsten Smith .............................................Texas BrianTech J. University, Camposano, KennethLubbock, L. Krysko, Texas Kevin 79409 M. Enge,([email protected]) Ellen M. Donlan, and Michael Granatosky 212 CONSERVATION ALERT n the night of. Wednesday,World’s Mammals in 14Crisis May ............................................................................................................................................................. 2014, 27 Texas three-week journey across South Africa 220 and Zimbabwe began Tech students tossed. More -
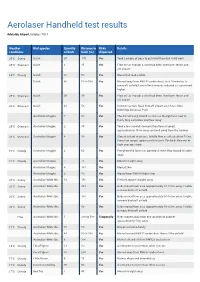
Aerolaser Handheld Test Results
Aerolaser Handheld test results Adelaide Airport October 2014 Weather Bird species Quantity Distance to Birds Details conditions of birds birds (m) dispersed 20°C Sunny Galah 20 170 Yes Took a couple of goes to get rid of them but it did work 20°C Overcast Galah 4 35 Yes Flew off as though a shot had been fired over fence and off airport 14°C Cloudy Galah 30 60 Yes Moved but took a while Galah 30 50 to 500 Yes Moved away from RWY12 undershoot, took 10 minutes to move off airfield, laser effectivenesds reduced as sun moved higher 20°C Overcast Galah 20 30 Yes Flew off as though a shot had been fired over fence and off airport 20°C Overcast Galah 40 50 Yes Instant reaction, flock flew off airport over fence intro Burbridge Business Park Australian Magpie 7 50 Yes The did not hang around as soon as the light was next to them, they carried on and flew away 20°C Overcast Australian Magpie 2 30 Yes Took a few seconds to react then flew at speed approximately 150m away and well away from the runway 20°C Overcast Australian Magpie 8 30 Yes Almost instant reactions. Initally flew as a flock about 100m. From that range I again used the laser. The birds flew out of sight over ops store 15°C Cloudy Australian Magpie 4 60 Yes Everytime the laser was pointed at them they moved straight away 15°C Cloudy Australian Magpie 7 70 Yes Moved straight away Australian Magpie 8 100 Yes Moved 24m Australian Magpie 2 50 Yes Moved from RWY05 flight strip 20°C Sunny Australian White Ibis 13 150 Yes Fled off airport straight away 20°C Sunny Australian White Ibis 250 Yes Birds moved from area approximately 50-100m away. -

Preliminary Studies on the Effect of Organochlorine Pesticides on Birds in Tanzania
IIIIIIIIIIII XA9743728 PRELIMINARY STUDIES ON THE EFFECT OF ORGANOCHLORINE PESTICIDES ON BIRDS IN TANZANIA A.S.M. UANI, J.M. KATONDO, J.M. MALULU Tropical Pesticides Research Institute, Arusha, Tanzania Abstract Preliminary studies to investigate the effects of organochlorine pesticides on birds was conducted in Lower Moshi, NAFCO West Kilimanjaro, Arusha seed farm. Tropical Pesticides Research Institute (TPRI) farms, Manyara ranch and areas around Lake Victoria as well as in the TPRI laboratory in Tanzania. Large quantities of the pesticides particularly DDT, endosulfan, dieldrin, lindane and toxaphene are still being applied against pests of cotton, coffee, maize, beans and other crops as well as disease vectors in the country. Several groups of birds including waterbirds, African Fish Eagles, Marabou storks, Oxpecker, ducks, etc. were found feeding, roosting and swimming in the water and exposed to other substances that were contaminated with organochlorine pesticides and were presumably at risk. Analytical results from the tissues of the African Fish Eagles collected from Lake Victoria areas showed that the kidneys were contaminated with p,p3- DDE and o,p3- DDE at levels of 0.4 ng g ' and 1.45 ng g'1 respectively. These organochlorine insecticides as well as P-HCH were also present in the brain and liver tissues. The levels of the organochlorine residues were well below the lethal and sublethal levels for bird raptors reported in the literature. 1. INTRODUCTION Organochlorine pesticides, including DDT, dieldrin, endosulfan, lindane and toxaphene, are still being used to control agricultural pests and vectors of human and animal diseases in developing countries in the tropical and subtropical regions. -

The Currawongs and the Magpies
Diru’wunan and Diru’wun The Currawongs and the Magpies www.dharawalstories.com Frances Bodkin Gawaian Bodkin-Andrews Illustrations By Lorraine Robertson THE CURRAWONGS AND THE MAGPIES Diru’wunan and Diru’wun A very, very long time ago, Warnan’nan, the People of the Raven became worried. 1 There had not been any rain from the time of the blooming of the Boo’kerrikin to the time of the blooming of the Ker’wan. The creeks and rivers were drying up, and even some of the deepest waterholes were become shallow enough for children to walk across. And at night, the Earth sang a song of thirst, pleading for water to soothe her parched skin. Oftentimes, the People of the Raven would look at the skies, watching and hoping that the Cloud Spirits would come and deliver their bur- den of rain. But the skies remained blue and cloudless. The People of the Raven went to Wiritjiribin, the People of the Lyrebird, to seek their advice, but found that they, too were suffering from lack of rain, although their sweet water came from the Earth herself, and not from the skies like the other peoples. The People of the Lyrebird were kind to the People of the Raven and invited them to take as much sweet water as they needed. The People of the Raven asked why the People of the Lyrebird were so kind when other Peoples had refused to help them. The People of the Lyrebird replied that a long, long time ago, the People of the Raven had provided their black feathered cloaks to the children of the Mull’goh, the Black Swan, and providing the sweet water was the repayment of a kin debt. -

CURRAWONGS, MAGPIES, RAVENS Do You Know the Difference?
Number 4 May 2010 CURRAWONGS, MAGPIES, RAVENS Do you know the difference? They wake you up early in the morning with their calls, they spread invasive weeds, disperse the contents of rubbish bins, consume baby birds for breakfast and swoop on you unexpectedly! However, they are all part of our shared environment. Pied Currawong Strepera graculina Size: 42–50 cm; Call: loud “currawong”, or deep croaks and wolf whistles. The Pied Currawong is a black bird that can be distinguished by its robust bill, yellow eyes, a white patch on its wing and white tip and underparts of its tail. Both sexes are similar, although the female is smaller and is often greyer on the underparts. The Pied Currawong is found only on the east coast of Australia. It inhabits rainforests, forests, woodlands, inland/coastal scrub, garbage tips, picnic grounds, parks and gardens. Pied Currawongs feed on small lizards, insects, berries, and small and young birds. Large prey items are stored in what’s called a “larder” (a tree fork or crevice) so prey can be eaten over a Pied Currawong period of time. Grey Currawongs Strepera versicolor are also found in the district. Australian Magpie Gymnorhina tibicen Size: 38–44 cm; Call: Rich mellow, organ-like carolling. The Australian Magpie is mostly black, with a prominent white nape (greyer in female), white shoulder and wing band, and a white rump and under tail. The eye colour of the Magpie is red to brown. It is found almost wherever there are trees and open areas. Magpies feed on worms, small reptiles, insects and their larvae, fruits and seeds, and will also take hand outs from humans, a practice certainly not to be encouraged. -

Canberra Bird Notes
ISSN 0314-8211 CANBERRA Volume 20 Number 2 BIRD June 1995 NOTES Registered by Australia Post - publication No NBH 0255 CANBERRA ORNITHOLOGISTS GROUP INC. P.O. Box 301, Civic Square, ACT 2608 Committee Members (1995) Work Home President Jenny Bounds -- 288 7802 Vice-President Paul Fennell -- 254 1804 Secretary Robin Smith -- 236 3407 Treasurer John Avery -- 281 4631 Member Gwen Hartican -- 281 3622 Member Bruce Lindenmayer 288 5957 288 5957 Member Pat Muller 298 9281 297 8853 Member Richard Schodde 242 1693 281 3732 Member Harvey Perkins -- 231 8209 -- 286 2624 Member Carol Macleay The following people represent Canberra Ornithologists Group in various ways although they may not be formally on the Committee: ADP Support Cedric Bear -- 258 3169 Australian Bird Count Chris Davey 242 1600 254 6324 Barren Grounds, Tony Lawson 264 3125 288 9430 Representative Canberra Bird Notes, David Purchase 258 2252 258 2252 Editor Assistant Editor Grahame Clark -- 254 1279 Conservation Council, 288 5957 288 5957 Representatives Bruce Lindenmayer Jenny Bounds -- 288 7802 Philip Veerman -- 231 4041 Exhibitions Coordinator (vacant) Field Trips, Jenny Bounds -- 288 7802 Coordinator Gang Gang, Harvey Perkins -- 231 8209 Editor Garden Bird Survey, Philip Veerman -- 231 4041 Coordinator Hotline Ian Fraser -- -- Librarian (vacant) Meetings, Barbara Allan -- 254 6520 Talks Coordinator (Continued inside back cover) TERRITORIALITY AND BREEDING SUCCESS OF AUSTRALIAN MAGPIES IN A CANBERRA SUBURB Chris Davey The Australian Magpie Gymnorhina tibicen is distributed throughout most of Australia. Within the Canberra area two subspecies occur, the black-backed G. t. tibicen and the white-backed G. t. hypoleuca. The black-backed subspecies is the one most commonly seen in the area. -

Tropical & Far North Queensland 9 Day Birding Tour
Bellbird Tours Pty Ltd PO Box 2008, BERRI SA 5343 AUSTRALIA Ph. 1800-BIRDING Ph. +61409 763172 www.bellbirdtours.com [email protected] Tropical & Far North Queensland 9 day birding tour Tropical Far North Queensland is without doubt one of known for such as Magnificent Riflebird, Frill-necked Monarch, Australia‟s top birding destinations. A variety of tropical Monarch, Blue-faced Parrot-finch, Lovely Fairy-wren, White- habitats including rainforests, palm-fringed beaches, White-streaked Honeyeater, Papuan Frogmouth, nesting Red mangrove-lined mudflats, savannahs and cool mountain Red Goshawks, Palm Cockatoo, Eclectus Parrot, Yellow-bellied ranges result in a bird diversity unparalleled elsewhere in bellied Kingfisher and many more, including of course the rare the country. rare Southern Cassowary. We‟ll have rare access to Golden- Commencing in Cairns, we‟ll bird the local area and head shouldered Parrot nesting sites; explore Lakefield National to well-known Kingfisher Park on the Atherton Tablelands, Park; we‟ll go night spotting for owls and mammals‟; and we‟ll then traverse the unique Cape York Peninsula. During this we‟ll spend two days in Iron Range National Park, one of tour we aim to find all Tropical Far North Queensland Australia‟s most important ecosystems. We‟ll also look for specialties. Expect over 200 species including all the mammals such as Bandicoot, Sugar Glider, Northern Striped endemics and other important birds this area is well- Striped Possum, Red-necked Wallaby and Tree Kangaroo. Read on below for the full -

Cooktown and District Bird Species
Cooktown and District Bird Species Southern Cassowary L White-necked Heron U Pacific Golden Plover C,M Pheasant Coucal C Yellow-spotted Honeyeater C Rufous Fantail U Emu R Eastern Great Egret C Grey Plover R,M Eastern Koel U,M,h Graceful Honeyeater U Grey Fantail U Australian Brush-turkey C Intermediate Egret U Red-capped Plover C Channel-billed Cuckoo U,M,h Bridled Honeyeater L,E Northern Fantail U Orange-footed Scrubfowl C Great-billed Heron L,R Lesser Sand Plover U,M Horsfield's Bronze-Cuckoo U,h Yellow-faced Honeyeater U Willie Wagtail U Brown Quail R Cattle Egret C Greater Sand Plover C,M Shining Bronze-Cuckoo U,h Varied Honeyeater L Torresian Crow C Indian Peafowl L,R Striated Heron U Black-fronted Dotterel U Little Bronze-Cuckoo C,h Yellow Honeyeater C Leaden Flycatcher C Magpie Goose U Pied Heron R,M Red-kneed Dotterel R Pallid Cuckoo U Brown-backed Honeyeater C Satin Flycatcher R,M Plumed Whistling-Duck R White-faced Heron C Masked Lapwing C Chestnut-breasted Cuckoo U Bar-breasted Honeyeater R Shining Flycatcher C Wandering Whistling-Duck U Little Egret C Comb-crested Jacana C Fan-tailed Cuckoo U Dusky Honeyeater C Restless Flycatcher R Radjah Shelduck U Eastern Reef Egret C Latham's Snipe U,M Brush Cuckoo C,h Scarlet Honeyeater U White-eared Monarch U Green Pygmy-goose C Nankeen Night-heron U Black-tailed Godwit R,M Oriental Cuckoo R,M Banded Honeyeater U Black-faced Monarch U Grey Teal U Glossy Ibis R Bar-tailed Godwit C,M Barking Owl U,h Brown Honeyeater R Black-winged Monarch L,M Pacific Black Duck C Australian White Ibis -

Dietary Breadth and Foraging Habitats of the White- Bellied Sea Eagle (Haliaeetus Leucogaster) on West Australian Islands and Coastal Sites
Dietary breadth and foraging habitats of the White- bellied Sea Eagle (Haliaeetus leucogaster) on West Australian islands and coastal sites. Presented to the Faculty of the Department of Environmental Science Murdoch University By Shannon Clohessy Bachelor of Science (Biological Sciences and Marine and Freshwater Management) Graduate Diploma of Science (Environmental Management) 2014 1 Declaration I declare that this thesis is a synthesis of my own research and has not been submitted as part of a tertiary qualification at any other institution. ……………………………………….. Shannon Clohessy 2014 2 Abstract This study looks at dietary preference of the Haliaeetus leucogaster in the Houtman Abrolhos and on coastal and near shore islands between Shark Bay and Jurien Bay. Prey species were identified through pellet dissection, which were collected from nests and feeding butcheries, along with prey remains and reference photographs. Data extracted from this process was compared against known prey types for this species. Potential foraging distances were calculated based on congeneric species data and feeding habits and used to calculate foraging habitat in the study sites and expected prey lists to compare against observed finds. Results were compared against similar studies on Haliaeetus leucogaster based in other parts of Australia. 3 Contents Figure list .................................................................................................................................. 6 Tables list ................................................................................................................................ -

Common Birds of Namibia and Botswana 1 Josh Engel
Common Birds of Namibia and Botswana 1 Josh Engel Photos: Josh Engel, [[email protected]] Integrative Research Center, Field Museum of Natural History and Tropical Birding Tours [www.tropicalbirding.com] Produced by: Tyana Wachter, R. Foster and J. Philipp, with the support of Connie Keller and the Mellon Foundation. © Science and Education, The Field Museum, Chicago, IL 60605 USA. [[email protected]] [fieldguides.fieldmuseum.org/guides] Rapid Color Guide #584 version 1 01/2015 1 Struthio camelus 2 Pelecanus onocrotalus 3 Phalacocorax capensis 4 Microcarbo coronatus STRUTHIONIDAE PELECANIDAE PHALACROCORACIDAE PHALACROCORACIDAE Ostrich Great white pelican Cape cormorant Crowned cormorant 5 Anhinga rufa 6 Ardea cinerea 7 Ardea goliath 8 Ardea pupurea ANIHINGIDAE ARDEIDAE ARDEIDAE ARDEIDAE African darter Grey heron Goliath heron Purple heron 9 Butorides striata 10 Scopus umbretta 11 Mycteria ibis 12 Leptoptilos crumentiferus ARDEIDAE SCOPIDAE CICONIIDAE CICONIIDAE Striated heron Hamerkop (nest) Yellow-billed stork Marabou stork 13 Bostrychia hagedash 14 Phoenicopterus roseus & P. minor 15 Phoenicopterus minor 16 Aviceda cuculoides THRESKIORNITHIDAE PHOENICOPTERIDAE PHOENICOPTERIDAE ACCIPITRIDAE Hadada ibis Greater and Lesser Flamingos Lesser Flamingo African cuckoo hawk Common Birds of Namibia and Botswana 2 Josh Engel Photos: Josh Engel, [[email protected]] Integrative Research Center, Field Museum of Natural History and Tropical Birding Tours [www.tropicalbirding.com] Produced by: Tyana Wachter, R. Foster and J. Philipp,