Vaccines and Related Biological Products Advisory Committee October 22, 2020 Meeting Presentation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

2024 7 Day Working Days Calendar
2024 7 Day Working Days Calendar The Working Day Calendar is used to compute the estimated completion date of a contract. To use the calendar, find the start date of the contract, add the working days to the number of the calendar date (a number from 1 to 1000), and subtract 1, find that calculated number in the calendar and that will be the completion date of the contract Date Number of the Calendar Date Monday, January 1, 2024 228 Tuesday, January 2, 2024 229 Wednesday, January 3, 2024 230 Thursday, January 4, 2024 231 Friday, January 5, 2024 232 Saturday, January 6, 2024 233 Sunday, January 7, 2024 234 Monday, January 8, 2024 235 Tuesday, January 9, 2024 236 Wednesday, January 10, 2024 237 Thursday, January 11, 2024 238 Friday, January 12, 2024 239 Saturday, January 13, 2024 240 Sunday, January 14, 2024 241 Monday, January 15, 2024 242 Tuesday, January 16, 2024 243 Wednesday, January 17, 2024 244 Thursday, January 18, 2024 245 Friday, January 19, 2024 246 Saturday, January 20, 2024 247 Sunday, January 21, 2024 248 Monday, January 22, 2024 249 Tuesday, January 23, 2024 250 Wednesday, January 24, 2024 251 Thursday, January 25, 2024 252 Friday, January 26, 2024 253 Saturday, January 27, 2024 254 Sunday, January 28, 2024 255 Monday, January 29, 2024 256 Tuesday, January 30, 2024 257 Wednesday, January 31, 2024 258 Thursday, February 1, 2024 259 Friday, February 2, 2024 260 Saturday, February 3, 2024 261 Sunday, February 4, 2024 262 Date Number of the Calendar Date Monday, February 5, 2024 263 Tuesday, February 6, 2024 264 Wednesday, February -

Pricing*, Pool and Payment** Due Dates January - December 2021 Mideast Marketing Area Federal Order No
Pricing*, Pool and Payment** Due Dates January - December 2021 Mideast Marketing Area Federal Order No. 33 Class & Market Administrator Payment Dates for Producer Milk Component Final Pool Producer Advance Prices Payment Dates Final Payment Due Partial Payment Due Pool Month Prices Release Date Payrolls Due & Pricing Factors PSF, Admin., MS Cooperative Nonmember Cooperative Nonmember January February 3 * February 13 February 22 December 23, 2020 February 16 ** February 16 February 17 Janaury 25 January 26 February March 3 * March 13 March 22 January 21 * March 15 March 16 March 17 February 25 February 26 March March 31 * April 13 April 22 February 18 * April 15 April 16 April 19 ** March 25 March 26 April May 5 May 13 May 22 March 17 * May 17 ** May 17 ** May 17 April 26 ** April 26 May June 3 * June 13 June 22 April 21 * June 15 June 16 June 17 May 25 May 26 June June 30 * July 13 July 22 May 19 * July 15 July 16 July 19 ** June 25 June 28 ** July August 4 * August 13 August 22 June 23 August 16 ** August 16 August 17 July 26 ** July 26 August September 1 * September 13 September 22 July 21 * September 15 September 16 September 17 August 25 August 26 September September 29 * October 13 October 22 August 18 * October 15 October 18 ** October 18 ** September 27 ** September 27 ** October November 3 * November 13 November 22 September 22 * November 15 November 16 November 17 October 25 October 26 November December 1 * December 13 December 22 October 20 * December 15 December 16 December 17 November 26 ** November 26 December January 5, 2022 January 13, 2022 January 22, 2022 November 17 * January 18, 2022 ** January 18, 2022 ** January 18, 2022 ** December 27 ** December 27 ** * If the release date does not fall on the 5th (Class & Component Prices) or 23rd (Advance Prices & Pricing Factors), the most current release preceding will be used in the price calculation. -

2021 7 Day Working Days Calendar
2021 7 Day Working Days Calendar The Working Day Calendar is used to compute the estimated completion date of a contract. To use the calendar, find the start date of the contract, add the working days to the number of the calendar date (a number from 1 to 1000), and subtract 1, find that calculated number in the calendar and that will be the completion date of the contract Date Number of the Calendar Date Friday, January 1, 2021 133 Saturday, January 2, 2021 134 Sunday, January 3, 2021 135 Monday, January 4, 2021 136 Tuesday, January 5, 2021 137 Wednesday, January 6, 2021 138 Thursday, January 7, 2021 139 Friday, January 8, 2021 140 Saturday, January 9, 2021 141 Sunday, January 10, 2021 142 Monday, January 11, 2021 143 Tuesday, January 12, 2021 144 Wednesday, January 13, 2021 145 Thursday, January 14, 2021 146 Friday, January 15, 2021 147 Saturday, January 16, 2021 148 Sunday, January 17, 2021 149 Monday, January 18, 2021 150 Tuesday, January 19, 2021 151 Wednesday, January 20, 2021 152 Thursday, January 21, 2021 153 Friday, January 22, 2021 154 Saturday, January 23, 2021 155 Sunday, January 24, 2021 156 Monday, January 25, 2021 157 Tuesday, January 26, 2021 158 Wednesday, January 27, 2021 159 Thursday, January 28, 2021 160 Friday, January 29, 2021 161 Saturday, January 30, 2021 162 Sunday, January 31, 2021 163 Monday, February 1, 2021 164 Tuesday, February 2, 2021 165 Wednesday, February 3, 2021 166 Thursday, February 4, 2021 167 Date Number of the Calendar Date Friday, February 5, 2021 168 Saturday, February 6, 2021 169 Sunday, February -

Advisory Opinion 20-03 on the Public Readiness
DEPARTMENT OF HEALTH & HUMAN SERVICES Office of the Secretary _____________________________________________________________________________________________________________________________________ The General Counsel Washington, D.C. 20201 ADVISORY OPINION 20-03 ON THE PUBLIC READINESS AND EMERGENCY PREPAREDNESS ACT AND THE SECRETARY’S DECLARATION UNDER THE ACT OCTOBER 22, 2020, AS MODIFIED ON OCTOBER 23, 2020 This Advisory Opinion addresses three vaccination-related issues that have arisen under the Public Readiness and Emergency Preparedness (PREP) Act, 42 U.S.C. § 247d-6d, as follows: 1. Does the PREP Act preempt state licensing laws that are less stringent than the federal standards under the Third Amendment to the Secretary’s March 10, 2020 PREP Act Dec- laration?1 2. May a state require a pharmacist to enter into a collaborative-practice agreement with a licensed physician as a condition of administering ACIP-recommended vaccines to chil- dren between ages 3 and 18? 3. Is epinephrine, when used to treat a severe acute vaccine reaction, a “covered counter- measure” within the meaning of the PREP Act? States or their sub-units may not impose any requirement that would prohibit or effectively prohibit activities authorized by the Secretary in a PREP Act declaration. Accordingly, state-licensing laws that are less stringent than those in the Secretary’s Declaration are not preempted, because they do not prohibit or effectively prohibit the activity in question—namely, vaccination. Conversely, any state or local law requiring a pharmacist to enter into a collaborative-practice agreement would be preempted if that requirement prohibits or effectively prohibits a pharmacist from ordering and administering vaccines as set forth in the Third Amendment and related issuances. -

FTE Reporting Deadlines 2019-20 Through 2022-23
FTE Reporting Deadlines 2019-20 through 2022-23 2019-20 2020-21 Survey 1 Survey 1 Survey Week: July 8-12, 2019 Survey Week: July 6-10, 2020 Due Date: July 26, 2019 Due Date: July 24, 2020 State Processing: July 22-September 13, 2019 State Processing: July 20-September 11, 2020 Final Update/Amendment Date: September 30, 2019 Final Update/Amendment Date: September 30, 2020 Survey 2 Survey 2 Survey Week: October 7-11, 2019 Survey Week: October 5-9, 2020 Due Date: October 18, 2019 Due Date: October 16, 2020 State Processing: October 14-November 1, 2019 State Processing: October 12- October 30, 2020 Final Update/Amendment Date: December 15, 2019 Final Update/Amendment Date: December 15, 2020 Survey 3 Survey 3 Survey Week: February 3-7, 2020 Survey Week: February 8-12, 2021 Due Date: February 14, 2020 Due Date: February 19, 2021 State Processing: February 10-February 28, 2020 State Processing: February 15-March 5, 2021 Final Update/Amendment Date: April 15, 2020 Final Update/Amendment Date: April 15, 2021 Survey 4 Survey 4 Survey Week: June 8-12, 2020 Survey Week: June 7-11, 2021 Due Date: July 2, 2020 Due Date: July 2, 2021 State Processing: June 22-July 10, 2020 State Processing: June 21-July 9, 2021 Final Update/Amendment Date: August 15, 2020 Final Update/Amendment Date: August 15, 2021 2021-22 2022-23 Survey 1 Survey 1 Survey Week July 12-16, 2021 Survey Week July 11-15, 2022 Due Date: July 30, 2021 Due Date: July 29, 2022 State Processing: July 26 – September 10, 2021 State Processing: July 25 – September 9, 2022 Final Update/Amendment -

2018 - 2019 Days of Rotation Calendar
2018 - 2019 DAYS OF ROTATION CALENDAR Day # Date Rotation Day Type Notes Day # Date Rotation Day Type Notes Saturday, October 13, 2018 Sunday, October 14, 2018 Monday, September 3, 2018 Holiday/Vaca Labor Day 27 Monday, October 15, 2018 Day 3 In Session 1 Tuesday, September 4, 2018 Day 1 In Session 28 Tuesday, October 16, 2018 Day 4 In Session 2 Wednesday, September 5, 2018 Day 2 In Session 29 Wednesday, October 17, 2018 Day 5 In Session 3 Thursday, September 6, 2018 Day 3 In Session 30 Thursday, October 18, 2018 Day 6 In Session 4 Friday, September 7, 2018 Day 4 In Session 31 Friday, October 19, 2018 Day 1 In Session Saturday, September 8, 2018 Saturday, October 20, 2018 Sunday, September 9, 2018 Sunday, October 21, 2018 Monday, September 10, 2018 Day Holiday/Vaca Rosh Hashanah 32 Monday, October 22, 2018 Day 2 In Session 5 Tuesday, September 11, 2018 Day 5 In Session 33 Tuesday, October 23, 2018 Day 3 In Session 6 Wednesday, September 12, 2018 Day 6 In Session 34 Wednesday, October 24, 2018 Day 4 In Session 7 Thursday, September 13, 2018 Day 1 In Session 35 Thursday, October 25, 2018 Day 5 In Session 8 Friday, September 14, 2018 Day 2 In Session 36 Friday, October 26, 2018 Day 6 In Session Saturday, September 15, 2018 Saturday, October 27, 2018 Sunday, September 16, 2018 Sunday, October 28, 2018 9 Monday, September 17, 2018 Day 3 In Session 37 Monday, October 29, 2018 Day 1 In Session 10 Tuesday, September 18, 2018 Day 4 In Session 38 Tuesday, October 30, 2018 Day 2 In Session Wednesday, September 19, 2018 Day Holiday/Vaca Yom Kippur 39 Wednesday, October 31, 2018 Day 3 In Session 11 Thursday, September 20, 2018 Day 5 In Session 40 Thursday, November 1, 2018 Day 4 In Session 12 Friday, September 21, 2018 Day 6 In Session 41 Friday, November 2, 2018 Day 5 In Session Saturday, September 22, 2018 Saturday, November 3, 2018 Sunday, September 23, 2018 Sunday, November 4, 2018 13 Monday, September 24, 2018 Day 1 In Session 42 Monday, November 5, 2018 Day 6 In Session 14 Tuesday, September 25, 2018 Day 2 In Session Tuesday, November 6, 2018 Prof Dev. -

Julian Date Cheat Sheet for Regular Years
Date Code Cheat Sheet For Regular Years Day of Year Calendar Date 1 January 1 2 January 2 3 January 3 4 January 4 5 January 5 6 January 6 7 January 7 8 January 8 9 January 9 10 January 10 11 January 11 12 January 12 13 January 13 14 January 14 15 January 15 16 January 16 17 January 17 18 January 18 19 January 19 20 January 20 21 January 21 22 January 22 23 January 23 24 January 24 25 January 25 26 January 26 27 January 27 28 January 28 29 January 29 30 January 30 31 January 31 32 February 1 33 February 2 34 February 3 35 February 4 36 February 5 37 February 6 38 February 7 39 February 8 40 February 9 41 February 10 42 February 11 43 February 12 44 February 13 45 February 14 46 February 15 47 February 16 48 February 17 49 February 18 50 February 19 51 February 20 52 February 21 53 February 22 54 February 23 55 February 24 56 February 25 57 February 26 58 February 27 59 February 28 60 March 1 61 March 2 62 March 3 63 March 4 64 March 5 65 March 6 66 March 7 67 March 8 68 March 9 69 March 10 70 March 11 71 March 12 72 March 13 73 March 14 74 March 15 75 March 16 76 March 17 77 March 18 78 March 19 79 March 20 80 March 21 81 March 22 82 March 23 83 March 24 84 March 25 85 March 26 86 March 27 87 March 28 88 March 29 89 March 30 90 March 31 91 April 1 92 April 2 93 April 3 94 April 4 95 April 5 96 April 6 97 April 7 98 April 8 99 April 9 100 April 10 101 April 11 102 April 12 103 April 13 104 April 14 105 April 15 106 April 16 107 April 17 108 April 18 109 April 19 110 April 20 111 April 21 112 April 22 113 April 23 114 April 24 115 April -
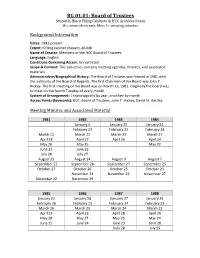
RG 01.01: Board of Trustees Stored in Black Filing Cabinets in HCC Archives Room # = Some Electronic Files; ! = Missing Minutes
RG 01.01: Board of Trustees Stored in Black Filing Cabinets in HCC Archives Room # = some electronic files; ! = missing minutes Background Information Dates: 1981-present Extent: 6 filing cabinet drawers, 86 MB Name of Creator: Members of the HCC Board of Trustees Language: English Conditions Governing Access: Unrestricted Scope & Content: This sub-series contains meeting agendas, minutes, and associated materials. Administrative/Biographical History: The Board of Trustees was formed in 1981 with the authority of the Board of Regents. The first Chairman of the Board was John T. Hickey. The first meeting of the Board was on March 13, 1981. Originally the board was to meet on the fourth Tuesday of every month. System of Arrangement: Chronologically by year, and then by month. Access Points (keywords): BOT, Board of Trustees, John T. Hickey, David M. Bartley Meeting Minutes and Associated Material 1981 1982 1983 1984 January 5 January 25 January 24 February 23 February 22 February 28 March 13 March 23 March 22 March 27 April 28 April 27 April 26 April 24 May 26 May 25 May 22 June 23 June 22 July 28 July 27 August 25 August 24 August 9 August 7 September 22 September 28 September 27 September 25 October 27 October 26 October 25 October 23 November 23 November 22 November 27 December 22 December 29 1985 1986 1987 1988 January 22 January 28 !January 27 January 26 February 26 February 25 February 24 February 23 March 26 March 25 March 24 March 22 April 23 April 22 April 28 April 26 May 28 May 27 May 26 May 24 June 25 June 24 June 23 June 28 -

Pay Date Calendar
Pay Date Information Select the pay period start date that coincides with your first day of employment. Pay Period Pay Period Begins (Sunday) Pay Period Ends (Saturday) Official Pay Date (Thursday)* 1 January 10, 2016 January 23, 2016 February 4, 2016 2 January 24, 2016 February 6, 2016 February 18, 2016 3 February 7, 2016 February 20, 2016 March 3, 2016 4 February 21, 2016 March 5, 2016 March 17, 2016 5 March 6, 2016 March 19, 2016 March 31, 2016 6 March 20, 2016 April 2, 2016 April 14, 2016 7 April 3, 2016 April 16, 2016 April 28, 2016 8 April 17, 2016 April 30, 2016 May 12, 2016 9 May 1, 2016 May 14, 2016 May 26, 2016 10 May 15, 2016 May 28, 2016 June 9, 2016 11 May 29, 2016 June 11, 2016 June 23, 2016 12 June 12, 2016 June 25, 2016 July 7, 2016 13 June 26, 2016 July 9, 2016 July 21, 2016 14 July 10, 2016 July 23, 2016 August 4, 2016 15 July 24, 2016 August 6, 2016 August 18, 2016 16 August 7, 2016 August 20, 2016 September 1, 2016 17 August 21, 2016 September 3, 2016 September 15, 2016 18 September 4, 2016 September 17, 2016 September 29, 2016 19 September 18, 2016 October 1, 2016 October 13, 2016 20 October 2, 2016 October 15, 2016 October 27, 2016 21 October 16, 2016 October 29, 2016 November 10, 2016 22 October 30, 2016 November 12, 2016 November 24, 2016 23 November 13, 2016 November 26, 2016 December 8, 2016 24 November 27, 2016 December 10, 2016 December 22, 2016 25 December 11, 2016 December 24, 2016 January 5, 2017 26 December 25, 2016 January 7, 2017 January 19, 2017 1 January 8, 2017 January 21, 2017 February 2, 2017 2 January -

Due Date Chart 201803281304173331.Xlsx
Special Event Permit Application Due Date Chart for Events from January 1, 2019 - June 30, 2020 If due date lands on a Saturday or Sunday, the due date is moved to the next business day Event Date 30 Calendar days 90 Calendar Days Tuesday, January 01, 2019 Sunday, December 02, 2018 Wednesday, October 03, 2018 Wednesday, January 02, 2019 Monday, December 03, 2018 Thursday, October 04, 2018 Thursday, January 03, 2019 Tuesday, December 04, 2018 Friday, October 05, 2018 Friday, January 04, 2019 Wednesday, December 05, 2018 Saturday, October 06, 2018 Saturday, January 05, 2019 Thursday, December 06, 2018 Sunday, October 07, 2018 Sunday, January 06, 2019 Friday, December 07, 2018 Monday, October 08, 2018 Monday, January 07, 2019 Saturday, December 08, 2018 Tuesday, October 09, 2018 Tuesday, January 08, 2019 Sunday, December 09, 2018 Wednesday, October 10, 2018 Wednesday, January 09, 2019 Monday, December 10, 2018 Thursday, October 11, 2018 Thursday, January 10, 2019 Tuesday, December 11, 2018 Friday, October 12, 2018 Friday, January 11, 2019 Wednesday, December 12, 2018 Saturday, October 13, 2018 Saturday, January 12, 2019 Thursday, December 13, 2018 Sunday, October 14, 2018 Sunday, January 13, 2019 Friday, December 14, 2018 Monday, October 15, 2018 Monday, January 14, 2019 Saturday, December 15, 2018 Tuesday, October 16, 2018 2019 Tuesday, January 15, 2019 Sunday, December 16, 2018 Wednesday, October 17, 2018 Wednesday, January 16, 2019 Monday, December 17, 2018 Thursday, October 18, 2018 Thursday, January 17, 2019 Tuesday, December 18, 2018 -

TENTATIVE SCHOOL CALENDAR 2020-2021 July '20 August '20 September '20 October '20 November '20 December '20 for up T
TENTATIVE SCHOOL CALENDAR 2020-2021 August 31 .................Building In-Service/Staff Work Days July ‘20 January ‘21 S M T W T F S S M T W T F S September 1-3 ..........Building In-Service/Staff 1 2 3 4 1 2 Work Days 5 6 7 8 9 10 11 3 4 5 6 7 8 9 September 7 .............No School/Labor Day September 8 .............First Day (1-8) 12 13 14 15 16 17 18 10 11 12 13 14 15 16 September 8 & 9 .......PS & K Orientation 19 20 21 22 23 24 25 17 18 19 20 21 22 23 September 10 ...........First Day (PS & K) 2 2 26 27 28 29 30 31 24 25 26 27 8 8 29 30 September 14-30 ......NWEA Testing 31 October 1-2...............NWEA Testing August ‘20 October 14 & 15 ........No School/Staff In-Service S M T W T F S February ‘21 October 16 ................No School October 19 ................No School/Staff In-Service 1 S M T W T F S October 20 ................Parent Conferences 2 3 4 5 6 7 8 1 2 3 4 5 6 (PS-8) 3:00 p.m.-7:00 p.m. 9 10 11 12 13 14 15 7 8 9 10 11 12 13 October 22 ................Parent Conferences 16 17 18 19 20 21 22 (PS-8) 3:00 p.m.-7:00 p.m. 14 15 16 17 18 19 20 23 24 25 26 27 28 29 21 22 23 24 25 26 27 November 12 ............Parent PM Pick Up/ 30 31 28 .................................Full Day November 12 ............End of 1st Quarter November 13 ............No School/Staff Work Day September ‘20 March ‘21 November 25-27 .......No School/Thanksgiving S M T W T F S S M T W T F S Break 1 2 3 4 5 1 2 3 4 5 6 6 7 8 9 10 11 12 December 23-31 .......No School/Winter Break 7 8 9 10 11 12 13 13 14 15 16 17 18 19 14 15 16 17 18 19 20 January 1 ..................No -

2021 Park Hours
Holiday World & Splashin' Safari 2021 Operating Calendar May, 2021 Holiday World Hours Splashin' Safari Hours Special Events Saturday, May 1 Holiday World & Splashin' Safari are closed Sunday, May 2 Holiday World & Splashin' Safari are closed Monday, May 3 Holiday World & Splashin' Safari are closed Tuesday, May 4 Holiday World & Splashin' Safari are closed Wednesday, May 5 Holiday World & Splashin' Safari are closed Thursday, May 6 Holiday World & Splashin' Safari are closed Friday, May 7 Holiday World & Splashin' Safari are closed Saturday, May 8 Platinum Season Pass Preview Day: Holiday World is open from 10 AM until 6 PM Splashin' Safari is Closed Platinum Season Passholder Party Sunday, May 9 Passholder Preview Day: Holiday World is open from 10 AM until 6 PM Splashin' Safari is Closed Monday, May 10 Holiday World & Splashin' Safari are closed Tuesday, May 11 Holiday World & Splashin' Safari are closed Wednesday, May 12 Holiday World & Splashin' Safari are closed Thursday, May 13 Holiday World & Splashin' Safari are closed Friday, May 14 Holiday World & Splashin' Safari are closed for a private outing Saturday, May 15 Holiday World is open from 10 AM until 4 PM Splashin' Safari is Closed Sunday, May 16 Holiday World is open from 10 AM until 4 PM Splashin' Safari is Closed Monday, May 17 Holiday World & Splashin' Safari are closed Tuesday, May 18 Holiday World & Splashin' Safari are closed Wednesday, May 19 Holiday World & Splashin' Safari are closed Thursday, May 20 Holiday World & Splashin' Safari are closed Friday, May 21 Holiday