Mineralogical, Geochemical, and Sedimentological Characteristics of Clay Deposits from Central Uganda and Their Applications George W.A
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

FAO Fisheries & Aquaculture
Food and Agriculture Organization of the United Nations Fisheries and for a world without hunger Aquaculture Department National Aquaculture Sector Overview Uganda I. Characteristics, Structure And Resources Of The Sector IV. Trends, Issues And Development a. Summary V. References b. History And General Overview a. Bibliography c. Human Resources b. Related Links d. Farming Systems Distribution And Characteristics e. Cultured Species f. Practices/Systems Of Culture II. Sector Performance a. Production b. Market And Trade c. Contribution To The Economy III. Promotion And Management Of The Sector a. The Institutional Framework b. The Governing Regulations c. Applied Research, Education And Training Characteristics, structure and resources of the sector Summary Uganda produces up to 15 000 tonnes of fish from aquaculture, including production from small-scale fish farmers, emerging commercial fish farmers and stocked community water reservoirs and minor lakes. There are an estimated 20 000 ponds throughout the country with an average surface area of 500 m² per pond. Production ranges between 1 500 kg per hectare per year for subsistence farmers to 15 000 kg per hectare per year for emerging commercial fish farmers. With improved market prices for fish, government intervention for increased production and stagnating supply from capture fisheries, aquaculture has begun to attract entrepreneurial farmers seeking to exploit the business opportunity provided by the prevailing demand for fish. This recent expansion in aquaculture has also resulted in the transformation of 20 percent to 30 percent of the smallholder subsistence ponds into profitable small-scale production units through developments in management as well as scale of production. -

Aquaculture Potential of Ornamental Fishes of Uganda
Aquaculture potential of ornamental fishes of Uganda Item Type monograph Authors Namulawa, V.T.; Ssekayi, J.; Namulemo, G. Publisher Aquaculture Research and Development Centre, National Fisheries Resources Research Institute Download date 29/09/2021 16:03:32 Link to Item http://hdl.handle.net/1834/35357 1Namulawa VT, 2Ssekayi J, 3Namulemo G 1Aquaculture Research & Development Centre, Kajjansi, P. O. Box 530, Kampala Uganda 2NAADS Coordinator, Kakiri Town Council, Wakiso District, P.O. Box 7218, Kampala Uganda 3National Fisheries Resources Research Institute, P. O. Box 343, Jinja Haplochromis nyererei Abstract Preliminary studies undertaken to investigate the availability of ornamental fish species in Uganda’s natural water systems, revealed significant abundance of coloured fishes in Uganda’s water systems including the Kyoga and Victoria Lake system. These species are able to breed in captivity and to feed on artificial diets in ponds and glass tanks. The species are attractive and are highly marketable. These Platytaeniodus sp. (Red tail) observations indicate the potential to culture ornamental fishes as away of diversifying the range of aquaculture species, a means to generate income and to improve livelihoods in Uganda. Introduction Ornamental Fish Trade is one of the most lucrative businesses in the world today, fetching close to USD 15 billion annually (Helfaman, 2007). This trade is led by countries such as Singapore, Hong Kong and Psammochromis riponanus Malaysia, with a diversity of ichthofauna as is the case in Uganda. This trade is popular and profitable because the unit value of ornamental fish is higher than that of food fish. In Uganda, several middle men have taken to collecting some ornamental fishes from Lakes Kyoga and Victoria, however, this is not ecologically sustainable. -

Mapping Uganda's Social Impact Investment Landscape
MAPPING UGANDA’S SOCIAL IMPACT INVESTMENT LANDSCAPE Joseph Kibombo Balikuddembe | Josephine Kaleebi This research is produced as part of the Platform for Uganda Green Growth (PLUG) research series KONRAD ADENAUER STIFTUNG UGANDA ACTADE Plot. 51A Prince Charles Drive, Kololo Plot 2, Agape Close | Ntinda, P.O. Box 647, Kampala/Uganda Kigoowa on Kiwatule Road T: +256-393-262011/2 P.O.BOX, 16452, Kampala Uganda www.kas.de/Uganda T: +256 414 664 616 www. actade.org Mapping SII in Uganda – Study Report November 2019 i DISCLAIMER Copyright ©KAS2020. Process maps, project plans, investigation results, opinions and supporting documentation to this document contain proprietary confidential information some or all of which may be legally privileged and/or subject to the provisions of privacy legislation. It is intended solely for the addressee. If you are not the intended recipient, you must not read, use, disclose, copy, print or disseminate the information contained within this document. Any views expressed are those of the authors. The electronic version of this document has been scanned for viruses and all reasonable precautions have been taken to ensure that no viruses are present. The authors do not accept responsibility for any loss or damage arising from the use of this document. Please notify the authors immediately by email if this document has been wrongly addressed or delivered. In giving these opinions, the authors do not accept or assume responsibility for any other purpose or to any other person to whom this report is shown or into whose hands it may come save where expressly agreed by the prior written consent of the author This document has been prepared solely for the KAS and ACTADE. -

THE UGANDA GAZETTE [13Th J Anuary
The THE RH Ptrat.ir OK I'<1 AND A T IE RKPt'BI.IC OF UGANDA Registered at the Published General Post Office for transmission within by East Africa as a Newspaper Uganda Gazette A uthority Vol. CX No. 2 13th January, 2017 Price: Shs. 5,000 CONTEXTS P a g e General Notice No. 12 of 2017. The Marriage Act—Notice ... ... ... 9 THE ADVOCATES ACT, CAP. 267. The Advocates Act—Notices ... ... ... 9 The Companies Act—Notices................. ... 9-10 NOTICE OF APPLICATION FOR A CERTIFICATE The Electricity Act— Notices ... ... ... 10-11 OF ELIGIBILITY. The Trademarks Act—Registration of Applications 11-18 Advertisements ... ... ... ... 18-27 I t is h e r e b y n o t if ie d that an application has been presented to the Law Council by Okiring Mark who is SUPPLEMENTS Statutory Instruments stated to be a holder of a Bachelor of Laws Degree from Uganda Christian University, Mukono, having been No. 1—The Trade (Licensing) (Grading of Business Areas) Instrument, 2017. awarded on the 4th day of July, 2014 and a Diploma in No. 2—The Trade (Licensing) (Amendment of Schedule) Legal Practice awarded by the Law Development Centre Instrument, 2017. on the 29th day of April, 2016, for the issuance of a B ill Certificate of Eligibility for entry of his name on the Roll of Advocates for Uganda. No. 1—The Anti - Terrorism (Amendment) Bill, 2017. Kampala, MARGARET APINY, 11th January, 2017. Secretary, Law Council. General N otice No. 10 of 2017. THE MARRIAGE ACT [Cap. 251 Revised Edition, 2000] General Notice No. -
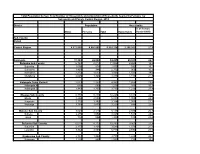
Population by Parish
Total Population by Sex, Total Number of Households and proportion of Households headed by Females by Subcounty and Parish, Central Region, 2014 District Population Households % of Female Males Females Total Households Headed HHS Sub-County Parish Central Region 4,672,658 4,856,580 9,529,238 2,298,942 27.5 Kalangala 31,349 22,944 54,293 20,041 22.7 Bujumba Sub County 6,743 4,813 11,556 4,453 19.3 Bujumba 1,096 874 1,970 592 19.1 Bunyama 1,428 944 2,372 962 16.2 Bwendero 2,214 1,627 3,841 1,586 19.0 Mulabana 2,005 1,368 3,373 1,313 21.9 Kalangala Town Council 2,623 2,357 4,980 1,604 29.4 Kalangala A 680 590 1,270 385 35.8 Kalangala B 1,943 1,767 3,710 1,219 27.4 Mugoye Sub County 6,777 5,447 12,224 3,811 23.9 Bbeta 3,246 2,585 5,831 1,909 24.9 Kagulube 1,772 1,392 3,164 1,003 23.3 Kayunga 1,759 1,470 3,229 899 22.6 Bubeke Sub County 3,023 2,110 5,133 2,036 26.7 Bubeke 2,275 1,554 3,829 1,518 28.0 Jaana 748 556 1,304 518 23.0 Bufumira Sub County 6,019 4,273 10,292 3,967 22.8 Bufumira 2,177 1,404 3,581 1,373 21.4 Lulamba 3,842 2,869 6,711 2,594 23.5 Kyamuswa Sub County 2,733 1,998 4,731 1,820 20.3 Buwanga 1,226 865 2,091 770 19.5 Buzingo 1,507 1,133 2,640 1,050 20.9 Maziga Sub County 3,431 1,946 5,377 2,350 20.8 Buggala 2,190 1,228 3,418 1,484 21.4 Butulume 1,241 718 1,959 866 19.9 Kampala District 712,762 794,318 1,507,080 414,406 30.3 Central Division 37,435 37,733 75,168 23,142 32.7 Bukesa 4,326 4,711 9,037 2,809 37.0 Civic Centre 224 151 375 161 14.9 Industrial Area 383 262 645 259 13.9 Kagugube 2,983 3,246 6,229 2,608 42.7 Kamwokya -

Opportunities for Affordable Construction in Uganda Using Locally Available Materials by Herbert Mwesigye Nuwagaba B.S
Opportunities for Affordable Construction in Uganda using Locally Available Materials By Herbert Mwesigye Nuwagaba B.S. Civil Engineering University of Illinois at Chicago, 2019 SUBMITTED TO THE DEPARTMENT OF CIVIL AND ENVIRONMENTAL ENGINEERING IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF ENGINEERING IN CIVIL AND ENVIRONMENTAL ENGINEERING AT THE MASSACHUSETTS INSTITUTE OF TECHNOLOGY May 2020 © 2020 Herbert Mwesigye Nuwagaba. All rights reserved. The author hereby grants to MIT permission to reproduce and to distribute publicly paper and electronic copies of this thesis document in whole or in part in any medium now known or hereafter created. Signature of Author: ………………………………………………………………………………. Herbert Mwesigye Nuwagaba Department of Civil and Environmental Engineering May 8, 2020 Certified by: ……………………………………………………………………………………….. Caitlin T. Mueller Ford International Career Development Professor Associate Professor of Civil and Environmental Engineering and Architecture Thesis Supervisor Accepted by: ………………………………………………………………………………………. Colette L. Heald Professor of Civil and Environmental Engineering Chair, Graduate Program Committee 2 Opportunities for Affordable Construction in Uganda using Locally Available Materials By Herbert Mwesigye Nuwagaba Submitted to the Department Of Civil and Environmental Engineering on May 8th, 2020 in Partial Fulfillment of the Requirements for the Degree of Master of Engineering in Structural Mechanics and Design. Abstract Uganda, like many other countries in the Global South, is in dire need of affordable housing. According to the 2017 Uganda National Household Survey, the number of poor Ugandans increased from 6.6 million in 2012/13 to 10 million in 2017 (Twinoburyo 2018). Uganda’s population growth is also the fourth highest in the world (The World Bank Group 2018). With the cost of manufacturing and purchasing construction materials like cement increasing due to scarcity of raw materials (Kamukama 2018), many Ugandans are unable to afford to construct homes. -
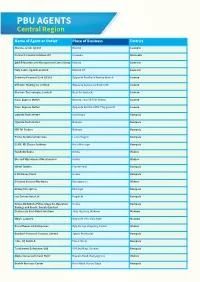
PBU AGENTS Central Region
PBU AGENTS Central Region Name of Agent or Outlet Place of Business District Abusha Junior (U) Ltd Bombo Luweero Parmer Financial Services Ltd Kapeeka Nakaseke Q&A Education and Management Consultancy Bombo Luweero Holy Saints Uganda Limited Bombo T/C Luweero Danortry Financial Link (U) Ltd Opposite PostBank Bombo Branch Luwero Dithcom Trading Co. Limited Opposite Centenary Bank ATM Luwero Glarison Technologies Limited Near the Barracks Luwero Nasu Express Outlet Bombo, near SR Fuel Station Luwero Nasu Express Outlet Opposite Bombo UPDF Play ground Luwero Uganda Post Limited Kyambogo Kampala Uganda Post Limited Nakawa Kampala NIK Tel Traders Nakawa Kampala Prime Secretarial Services Luzira Stage 6 Kampala ESSIE. RE Classic Fashions Biina Mutungo Kampala Nandudu Babra Kireka Wakiso Blessed Mina General Merchandise Kireka Wakiso Niktel Traders Pioneer Mall Kampala 6 GS Money Point Kireka Kampala Cinemax General Hardware Bweyogerere Wakiso Ariong Enterprises Mutungo Kampala Isip Connections Ltd Bugolobi Kampala Kireka BodaBoda Police Stage Co-Operative Kireka Kampala Savings and Credit Society Limited Chelanasta Cash Point Solutions Jinja Highway, Mukono Mukono Milly's Laundry Opposite UCU Main Gate Mukono Rana Phones 83 Enterprises Opp Kirinya Shopping Centre Wakiso Boadaph Financial Services Limited Sports Pro Hostel. Kampala Jeba (U) Limited Cwa 2 Street Kampala Tusaboomu Enterprises Ltd URA building, Nakawa Kampala Alpha Immanuel's Cash Point Najeera Road, Bweyogerere Wakiso Beulah Business Center Biina Road, Kunya Stage Kampala Kyobe -

National Guidelines: Managing Healthcare Waste Generated from Safe Male Circumcision Procedures | I
AUGUST 2013 This publication was made possible through the support of the U.S. President’s Emergency Plan for AIDS Relief (PEPFAR) through the U.S. Agency for International Development under contract number GHH-I-00-07-00059-00, AIDS Support and Technical Assistance Resources (AIDSTAR-One) Project, Sector I, Task Order 1. Uganda National Guidelines: Managing Healthcare Waste Generated from Safe Male Circumcision Procedures | i Acronyms ACP AIDS Control Programme HCW health care waste HCWM health care waste management IP implementing partner MoH Ministry of Health NDA National Drug Authority NEMA National Environmental Management Authority PEPFAR U.S. President’s Emergency Plan for AIDS Relief PHC primary health center PMTCT prevention of mother-to-child transmission POP persistent organic pollutants SMC safe male circumcision STI sexually transmitted infections UAC Uganda AIDS Commission UNBOS Uganda National Bureau of Standards USG U.S. Government WHO World Health Organization ii | Uganda National Guidelines: Managing Healthcare Waste Generated from Safe Male Circumcision Procedures Table of Contents Acronyms ......................................................................................................................................................... ii Preface ............................................................................................................................................................. v 1.0 BACKGROUND ......................................................................................................................................... -

Housing Investment Chronicles in Uganda Six Individual Household Stories March 2019
Housing Investment Chronicles in Uganda Six Individual Household Stories March 2019 This paper was prepared with the support of: Housing Investment Chronicles Uganda March 2019 Executive Summary – Six Individual Household Stories The Centre for Affordable Housing Finance in Africa (CAHF) launched its Housing Investment Chronicles (HIC) project in November 2016 with the aim of stimulating a greater understanding of low and middle income housing needs and investment journeys. The Housing Investment Chronicles document the housing investment journeys of participants over time. The goal of the project is to enhance increased and in-depth understanding of how households invest in housing, and how they navigate various systems, leverage existing opportunities and overcome diverse challenges to meet their housing needs. Additionally, the research aspires to stimulate long and short-term investment and to encourage down-market penetration of affordable and responsive housing finance packages. Last but not least, the project draws from these individual narratives to inform stakeholders and generate recommendations to decision-makers in both the public and private sphere. The Uganda Housing Investment Chronicles project was coordinated and carried out by a team of staff from Shelter and Settlements Alternatives, supported by an external consultant, in collaboration with different housing cooperative groups working in the Greater Kampala Metropolitan Area. The study was carried out in March and April 2018, with a 680-household survey conducted across the breadth of the Greater Kampala Metropolitan Area (GKMA) in the towns of Entebbe, Kajjansi, Ggaba, Maya, Kiira, Nansana, Ganda, Gayaza, Mukono and Buyale. The survey was supplemented by three focus group discussions with members from various housing cooperative unions from Mukono and Wakiso Districts, as well as with key players in the housing and financial sectors. -

Classified Adverts
CLASSIFIED ADVERTS NEW VISION, Wednesday, September 1, 2010 35 PROPERTIES MAILO “With Trust & Honesty” LAND SITES HOSSANA REAL RABAI REAL ESTATES ...your place to call home LIMITED ESTATES LTD .....we settle you Well surveyed plots with HERITAGE SITES LTD GLORYLAND REAL ready Mailo land titles. 1. KAWANDA - Bombo ESTATES LTD. 'We shall not bend the truth to PLANNED PLOTS WITH CANAAN SITES 1. GAYAZA-ZIROBWE RD. make a sale' PRIVATE MAILO LAND WELL PLANNED AND Road with good view Well planned plots with Mailo 50 x 100ft - 2.5m 50 x 100ft - 7m Dealers in: Buying & Selling of TITLES ORGANISED ESTATES 100 x 100ft - 5m land titles, water and power. Land, Land Documentation, WITH MAILO LAND 100 x 100ft - 14m PLOT 20 OLD K’LA NEAR ST MATIA 1. ENTEBBE ROAD 1 Acre at 15m 1. KASANGATI - near town Sites Manager & Land TITLES,WATER, POWER MULUMBA CHURCH KISUBI -WAMALA- 2. GAYAZA-ZIROBWE 2. KAWUKU, ENTEBBE (1km) the estate has a Surveying. 1. VICTORIA GARDENS 1 Km from tarmac “FIRST STEP TO NEW HOME” BUZI on the shores of Lake ROAD - with lake developed neighbourhood 50 x 100ft - 4.5m view on the main road 1. Kawuku Entebbe Road. GET A HOME IN OUR WELL 50 x 100ft - 6.5m Victoria 1.5km from tarmac with a good view of PLANNED ESTATES: 60 x 100ft - 20m 100 x 100ft - 9m 50 x 100ft = 12m Kasangati Town and With beautiful view of SEVERAL PLOTS WITH 100 x 100ft - 13m 70 x 100ft - 24m 3. BULOBA-MITYANA RD. the Lake 2km from main road 100 x 100ft = 24m Gayaza Town MAILO LAND TITLES AT 2. -

Annual Report 2012/2013 2
Annual Report 2012 1 Contents CCOORSRSUU Restoring Ability, Giving Hope. Annual Report 2012/2013 www.corsu.or.ug 2 Contents General Assembly Message from CoRSU Board President 3 Mr. Aloysius K. Bakkidde President Annual summary 4 Dr. Alice Nganwa Introduction 6 Mr. Jean Pierre Lequeux CoRSU Services: Surgery and Rehabilitation 8 Mr. William Byaruhanga Orthopaedic Rehabilitation Services 9 Dr. Sam Orochi Orach Plastic and Reconstructive Services 12 Mr. Ben Kayongo Male Therapy Services 14 Dr. Emmanuel B.K. Luyirika Orthopaedic Workshop 16 Mr. Victor B. O. Odongo Nutrition Rehabilitation 18 Mr. Jack M. Wavamunno Outreach Services 19 Dr. F. F. Tusubira Community Based Rehabilitation 20 Staff at CoRSU 22 Training 23 The Executive Board Visiting Specialists 24 Working with Partner organizations 25 Mr. Aloysious K. Bakkidde President Our Supporters 27 Dr. Alice Nganwa Vice-president Finances 28 Mr. Jean Pierre Lequeux Treasurer Way Forward: Aims for 2013 30 Dr. Emmanuel B.K. Luyirika Mr. Ben Kayongo Male Mr. Simon Bridger Secretary Mr. Victor B. Odongo Abbreviations Mr. Jack M. Wavamunno ACHERU Afaayo Child Health and Education Rehabilitation Unit Senior Management AGM Annual General Meeting CBM Christian Blind Mission / Christoffel-Blindenmission Team: CBR Community Based Rehabilitation Mr. Simon Bridger CEO CEO Chief Executive Officer Mr. Matthias Widmaier Admin. Director CoRSU Comprehensive Rehabilitation Services in Uganda Dr. Antonio Loro Medical Director COSECSA College of surgeons of East, Central and Southern Mr. Moses Kiwanuka Head -

CURRICULUM VITAE PROFESSOR ANDREW PETERS YIGA, (Phd)
CURRICULUM VITAE PROFESSOR ANDREW PETERS YIGA, (PhD) A. PERSONAL BACKGROUND Date of Birth : October 20, 1950 Place of Birth : Masaka – Uganda Nationality : Ugandan Passport Number : B0612263 Postal Address : P.O. Box 237, Entebbe, Uganda Mobile : 0772 -446510 Emails : [email protected]/ [email protected] B. CURRENT EMPLOYMENT 1. Appointed Academic Registrar of Nkumba University - February 1, 2018. Duties and Responsibilities “The duties and responsibilities in accordance to Section 51 of the University Charter Notice 2007 are: a) The Academic Registrar shall assist the Vice Chancellor in the administration and organization of academic matters including admissions, undergraduate and postgraduate studies, examinations, research and publications, staff development and such other responsibilities as may be assigned to him/her by the Vice Chancellor. b) The Academic Registrar shall be the Secretary to the Senate and all its Committees. c) The Academic Registrar shall be responsible for the affairs of the Alumni Association. d) The Academic Registrar shall be reporting to the Vice Chancellor.” 2. Promoted to the Rank of “Professor of Art and Design Education” in Nkumba University – October 19, 2018. Duties and Responsibilities “To hold the rank of a Professor in a University is a mark of academic growth and distinction. It is a position of leadership not only in academic pursuits but also in many other respects in the community. Therefore, in addition to your administrative role as the Academic Registrar, you will be required: a) To supervise Postgraduate Research for students. b) To promote knowledge in your field not only through teaching and other related activities but also through your original research and postgraduate work.