Impact of Lithium on Radioactive Iodine Therapy for Hyperthyroidism
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Actress Ashley Clements
Community Community Swasthi Japanese Academy for artist P7 Excellence P20 Masakatsu promises to focus Sashie’s solo on honing fine exhibition will go on talents to their display at the Anima utmost limits. Gallery on May 9. Friday, April 29, 2016 Rajab 22, 1437 AH DOHA 25°C—38°C TODAY PUZZLES 14 & 15 LIFESTYLE/HOROSCOPE 16 JJane,ane, a hhurricaneurricane Centuries later, the famous writer continues to inspire books, clubs, movies and more. P2-3 COVER STORY 2 GULF TIMES Friday, April 29, 2016 COMMUNITY COVER STORY No plain Jane The writer’s fiction is the basis for many film and TV adaptations as well as the template for countless PRAYER TIME rom-com movies or romance novels. Also, unlikely Fajr 3.38am Shorooq (sunrise) 5.00am mashups into the Austen universe, writes Tom Beer Zuhr (noon) 11.31am Asr (afternoon) 3.00pm Maghreb (sunset) 6.05pm Isha (night) 7.35pm USEFUL NUMBERS Emergency 999 Worldwide Emergency Number 112 Kahramaa – Electricity and Water 991 Ooredoo Telephone Assistance 111 Local Directory 180 International Calls Enquires 150 Time 141, 140 Doha International Airport 40106666 Labor Department 44508111, 44406537 Medical Commission 44679111 Mowasalat Taxi 44588888 Qatar Airways 44496000 Weather Forecast 44656590 Hamad Medical Corporation 44392222 44393333 Qatar General Electricity and Water Corporation 44845555 44845464 Primary Health Care Corporation 44593333 44593363 Qatar Assistive Technology Centre 44594050 Qatar News Agency 44450205 44450333 Q-Post – General Postal Corporation 44464444 Qatar University 44033333 ote Unquo Qu te The pen is mightier than the sword if the sword ane Austen (1775-1817) Metropolitan High School, dubs her Cher Horowitz is very short, and the pen is isn’t just for English majors Whit Stillman’s indie-fi lm gem from (Alicia Silverstone) and puts her in a anymore. -

P6participated P20 Innovators in the Fourth in the World, Is Driven Race of the Latest by the Self-Devised Edition of Qatar Ecosystem That Running Series
Community Community Over 100 Sweden, one runners of the top P6participated P20 innovators in the fourth in the world, is driven race of the latest by the self-devised edition of Qatar ecosystem that Running Series. nurtures creativity. Monday, May 9, 2016 Sha’baan 2, 1437 AH DOHA 29°C—40°C TODAY LIFESTYLE/HOROSCOPE 13 PUZZLES 14 & 15 Watch COVER STORY out! Hospital discharge is one of the most dangerous periods for patients. P4-5 DIVERSE: Mubarak al-Malik’s work. The al-Markhiya gallery is showcasing the works of three diverse Qatari artists. Details on Page 9 2 GULF TIMES Monday, May 9, 2016 COMMUNITY ROUND & ABOUT PRAYER TIME Fajr 3.29am Shorooq (sunrise) 4.53am Zuhr (noon) 11.30am Asr (afternoon) 2.58pm Maghreb (sunset) 6.10pm Isha (night) 7.40pm USEFUL NUMBERS Just The 3 Of Us live under the same roof as they both deal with their sense of GENRE: Comedy, Romance responsibility and their yearning for security. Uno is motivated by Emergency 999 CAST: John Lloyd Cruz, Jennylyn Mercado, Richard Yap his sense of family while CJ is governed by an unconditional love Worldwide Emergency Number 112 DIRECTION: Cathy Garcia-Molina that should put her fi rst above everything else. The unlikely thing Kahramaa – Electricity and Water 991 SYNOPSIS: Just The 3 Of Us is centered on an unlikely love that binds Uno and CJ together paves the way for them to fi nd in Ooredoo Telephone Assistance 111 story between polar opposites Uno (Cruz) and CJ (Mercado) – each other the kind of love they never thought they needed. -

The Journal of International Communication Film Remakes As
This article was downloaded by: [Mr C.S.H.N. Murthy] On: 08 January 2015, At: 09:46 Publisher: Routledge Informa Ltd Registered in England and Wales Registered Number: 1072954 Registered office: Mortimer House, 37-41 Mortimer Street, London W1T 3JH, UK The Journal of International Communication Publication details, including instructions for authors and subscription information: http://www.tandfonline.com/loi/rico20 Film remakes as cross-cultural connections between North and South: A case study of the Telugu film industry's contribution to Indian filmmaking C.S.H.N. Murthy Published online: 13 Nov 2012. To cite this article: C.S.H.N. Murthy (2013) Film remakes as cross-cultural connections between North and South: A case study of the Telugu film industry's contribution to Indian filmmaking, The Journal of International Communication, 19:1, 19-42, DOI: 10.1080/13216597.2012.739573 To link to this article: http://dx.doi.org/10.1080/13216597.2012.739573 PLEASE SCROLL DOWN FOR ARTICLE Taylor & Francis makes every effort to ensure the accuracy of all the information (the “Content”) contained in the publications on our platform. However, Taylor & Francis, our agents, and our licensors make no representations or warranties whatsoever as to the accuracy, completeness, or suitability for any purpose of the Content. Any opinions and views expressed in this publication are the opinions and views of the authors, and are not the views of or endorsed by Taylor & Francis. The accuracy of the Content should not be relied upon and should be independently verified with primary sources of information. Taylor and Francis shall not be liable for any losses, actions, claims, proceedings, demands, costs, expenses, damages, and other liabilities whatsoever or howsoever caused arising directly or indirectly in connection with, in relation to or arising out of the use of the Content. -

Pkt-Div-2011-12-Unpaid
SLNO FOLIO/DEM AT ID NAM E AD1 AD2 AD3 AD4 P INC OD DWNO M IC R DIV AM OU NT 1 000064 MR. OTTO GROB MERCHANT NESSLAN SW ITEZERLAND SW ITZERLAND 2883 2893 1740.00 2 000075 SMT. LALITHA KARU NAKAR NO.4.GAJAPATHY STREET KILPAU K CHENNAI. MADRAS - 600 010 600010 1852 1862 560.00 3 000085 SRI. S S MANI K.R.IENGR ROAD (BR) TEYNAMPET PO CHENNAI. MADRAS - 600 018 600018 1911 1921 1005.00 4 000123 MRS. M A B DA.COSTA AZAREDO RU A DE S.JOAQU IU N BORDA MARGAO GOA GOA 284 291 90.00 5 000127 MISS M A E DA COSTA RU ADE ABADE FARIA.MARGAO. GOA GOA 285 292 6260.00 6 000148 MISS AMINA ESSOP MIA C/O.TRANSVAAL INVESTMENT CO.LTD.. 65.BREE ST..NEW TOW N JOHANNESBU RG -2001 (REPU BLIC OF S.A.) 2986 2996 2075.00 7 000150 SHRI KN M PR VEERAPPA CHETTIAR BANKER. NATARAJAPU RAM PO.. RAMNAD DISTRICT RAMNAD DIST. 286 293 35.00 8 000166 SRI RAGHU NATHAN (EXECU TOR TO THE ESTATE 52 K.R.IYENGAR ROAD ALW ARPET CHENNAI. MADRAS - 600 018 600018 1912 1922 50.00 9 000177 SRI. S G NADKARNY BLOCK NO.3A. PLOT NO.80 DR.ANNIE BEASANT ROAD W ORLI, MU MBAI. BOMBAY 400 018. 400018 918 925 555.00 10 000202 RAO SAHIB M.G. RAMACHANDER ROW PU DU KOTTAI PU DU KOTTAI 288 295 4175.00 11 000219 MR. MAHOMED IBRAHIM KHATRI 45. "DANLAT". NEAR COLABA P.O.. MU MBAI. BOMBAY - 400 005 400005 782 789 3750.00 12 000220 MR. -

Directory of Participating Providers
Directory of Participating Providers For Members of HMSA's Plan for Federal Employees 2021 This directory is current as of August 2020 Aloha HMSA Federal Plan Members, The doctors and other health care providers you choose can affect how much you pay out of pocket for their services. Where to get care If you get care from participating providers, you'll only pay copayments and/or a coinsurance and you won't have to file claims. If you use our point-of-service program, you can also get care from non-plan providers, but it'll cost you more. We consider the following when evaluating and approving providers: • Is the provider accredited by a recognized accrediting agency? • Is the provider appropriately licensed? • Is the provider certified by the proper government authority? • Are the services performed within the lawful scope of the provider’s respective licensure, certification, and/or accreditation? Participating providers Participating providers are physicians and other health care professionals in our service area that we contract with to provide services to our members. We credential plan providers according to national standards. To receive plan benefits for out-of-state services under this plan, the services must be provided by a Blue Cross and Blue Shield participating provider. We list plan providers in this directory. Non-plan providers Non-plan providers are physicians and other health care professionals who are not under contract with this plan. For out-of-state services under this plan, non-plan provider benefits are applied for services performed by non-Blue Cross and Blue Shield Association programs. -
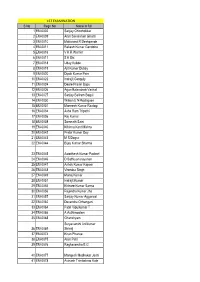
1ST EXAMINATION S No Regn No Name in Full 1 EM-0002 Sanjay
1ST EXAMINATION S No Regn No Name in full 1 EM-0002 Sanjay Chincholikar 2 EM-0009 Arun Savalaram Ghanti 3 EM-0010 Makarand R Deshpande 4 EM-0011 Rakesh Kumar Gandotra 5 EM-0016 V K R Warrier 6 EM-0017 S K Dix 7 EM-0018 Uday Kukde 8 EM-0019 Ajit Kumar Dubey 9 EM-0020 Dipak Kumar Pain 10 EM-0022 Indrajit Ganguly 11 EM-0024 Deore Pravin Bapu 12 EM-0026 Arjun Balasaheb Vavhal 13 EM-0027 Sanjay Baliram Bagul 14 EM-0030 Wilson E N Rodrigues 15 EM-0031 Muneesh Kumar Rastogi 16 EM-0034 Asha Ram Tripathi 17 EM-0036 Raj Kumar 18 EM-0038 Somnath Saini 19 EM-0040 Krishna Kant Mishra 20 EM-0042 Prabir Kumar Dey 21 EM-0043 M S Dogra 22 EM-0044 Bijay Kumar Sharma 23 EM-0045 Awadhesh Kumar Pachori 24 EM-0046 D Sathiyanarayanan 25 EM-0047 Ashok Kumar Kapoor 26 EM-0048 Virendra Singh 27 EM-0049 Manoj Kumar 28 EM-0051 Indrajit Kumar 29 EM-0055 Kishore Kumar Sarma 30 EM-0056 Rajendra Kumar Jha 31 EM-0057 Sanjay Kumar Aggarwal 32 EM-0062 Devendra Chhangani 33 EM-0064 Patel Vipulkumar T 34 EM-0066 A Authimoolam 35 EM-0068 Ghanshyam Suryavanshi Anilkumar 36 EM-0069 Shivaji 37 EM-0074 Kiran Phanse 38 EM-0075 Arun Patil 39 EM-0076 Raghavendra B G 40 EM-0077 Mangesh Madhukar Joshi 41 EM-0078 Avinash Trimbakrao Kale 42 EM-0079 Jaisankar G 43 EM-0080 Krishnan A G 44 EM-0082 Akash Agarwal 45 EM-0085 Umeshwar Vishwakarma 46 EM-0086 Upendra Manohar Bokare 47 EM-0087 Nihar Ranjan Mishra 48 EM-0088 Dharmendra Kumar Gupta Mahendrakumar Nathalal 49 EM-0089 Patel 50 EM-0090 Sudhakar R 51 EM-0092 Rajeev A S 52 EM-0093 Piyush Misra 53 EM-0094 Diwakar Misra 54 EM-0095 Chinmoy -

TAMIL CINEMA Tamil Cinema
TAMIL CINEMA Tamil cinema (also known as Cinema of Tamil Nadu, the Tamil film industry or the Chennai film industry) is the film industry based in Chennai, Tamil Nadu, India, dedicated to the production of films in the Tamil language. It is based in Chennai's Kodambakkam district, where several South Indian film production companies are headquartered. Tamil cinema is known for being India's second largest film industry in terms of films produced, revenue and worldwide distribution,[1] with audiences mainly including people from the four southern Indian states of Tamil Nadu, Kerala, Andra Pradesh, and Karnataka. Silent films were produced in Chennai since 1917 and the era of talkies dawned in 1931 with the film Kalidas.[2] By the end of the 1930s, the legislature of the State of Madras passed the Entertainment Tax Act of 1939.[3] Tamil cinema later had a profound effect on other filmmaking industries of India, establishing Chennai as a secondary hub for Telugu cinema, Malayalam cinema, Kannada cinema, and Hindi cinema. The industry also inspired filmmaking in Tamil diaspora populations in other countries, such as Sri Lankan Tamil cinema and Canadian Tamil cinema.[6] Film studios in Chennai are bound by legislation, such as the Cinematography Film Rules of 1948,[7] the Cinematography Act of 1952,[8] and the Copyright Act of 1957.[9] Influences Tamil cinema has been impacted by many factors, due to which it has become the second largest film industry of India. The main impacts of the early cinema were the cultural influences of the country. The Tamil language, ancient than the Sanskrit, was the medium in which many plays and stories were written since the ages as early as the Cholas. -

Annual Return – MGT-7
FORM NO. MGT-7 Annual Return [Pursuant to sub-Section(1) of section 92 of the Companies Act, 2013 and sub-rule (1) of rule 11of the Companies (Management and Administration) Rules, 2014] Form language English Hindi Refer the instruction kit for filing the form. I. REGISTRATION AND OTHER DETAILS (i) * Corporate Identification Number (CIN) of the company L14102TG1991PLC013299 Pre-fill Global Location Number (GLN) of the company * Permanent Account Number (PAN) of the company AABCP2100Q (ii) (a) Name of the company POKARNA LIMITED (b) Registered office address 1ST FLOOR, 105,SURYA TOWERS, SECUNDERABAD. A.P Telangana 500003 India (c) *e-mail ID of the company [email protected] (d) *Telephone number with STD code 04027897722 (e) Website www.pokarna.com (iii) Date of Incorporation 09/10/1991 (iv) Type of the Company Category of the Company Sub-category of the Company Public Company Company limited by shares Indian Non-Government company (v) Whether company is having share capital Yes No (vi) *Whether shares listed on recognized Stock Exchange(s) Yes No Page 1 of 15 (a) Details of stock exchanges where shares are listed S. No. Stock Exchange Name Code 1 B.S.E Limited 1 2 National Stock Exchange of India 1,024 (b) CIN of the Registrar and Transfer Agent U72400TG2017PTC117649 Pre-fill Name of the Registrar and Transfer Agent KFIN TECHNOLOGIES PRIVATE LIMITED Registered office address of the Registrar and Transfer Agents Selenium, Tower B, Plot No- 31 & 32, Financial District, Nanakramguda, Serilingampally (vii) *Financial year From date 01/04/2020 (DD/MM/YYYY) To date 31/03/2021 (DD/MM/YYYY) (viii) *Whether Annual general meeting (AGM) held Yes No (a) If yes, date of AGM (b) Due date of AGM 30/09/2021 (c) Whether any extension for AGM granted Yes No (f) Specify the reasons for not holding the same II. -

Craig to Earn 50 Mn Pounds for His Role
17 MONDAY, MAY 28, 2018 PARMANU Wadi Al Sail 8.30, 11.15 Pm (3D): 8.00, 10.45 Pm Cineco (20) 10.30, 12.45, 3.00, 5.15, 7.30, 9.45, 12.00 Mn ( HINDI/ ACTION/ DRAMA/ HISTORY ) John Abraham, Boman Irani, Diana Penty INTERROGATION A Wrinkle In Time DANA CINEMA 10:45,13:15,15:45,18:15,20:45,23:15 ( BIOGRAPHY/ DRAMA/ SPORT ) (Pg-13) (Adventure/Fantasy) Margot Robbie, Sebastian Stan, Allison Janney Storm Reid, Oprah Winfrey, Reese Witherspoon Cineco (20) 12.45, 3.30, 6.15, 9.00, 11.45 Pm DANA CINEMA 11:00,12:45,14:30,16:15,18:00,19:45,21:3 Cineco (20) 12.00, 2.15, 4.30, 6.45, 9.00, 11.15 Craig to earn Seef (I) 12.30, 3.15, 6.00, 8.45, 11.30 Pm 0,23:15,01:00 Saar 8.30, 11.15 Pm Cineco (20) 11.45 Am, 1.45, 3.45, 5.45, 7.45, 9.45, 11.45 Pm Life Of The Party Wadi Al Sail 8.15, 11.00 Pm 11.30 Am, 1.30, 3.30, 5.30, 7.30, 9.30, 11.30 Pm (15+) (Comedy) Seef (Ii) Melissa Mccarthy, Gillian Jacobs, Julie Bowen KAALI Wadi Al Sail 9.00, 11.00 Pm Cineco (20) 12.15, 2.30, 4.45, 7.00, 9.15, 11.30 50 mn pounds (TAMIL / ACTION ) NELA TICKET Vijay Antony, Sunaina, Anjali Breaking In ( TELUGU / DRAMA ) DANA CINEMA 10:30,15:45,21:00 Ravi Teja, Malavika Sharma, Brahmanandam (Pg-15) (Thriller/Crime) Gabrielle Union, Billy Burke, Richard Cabral NADIGAIYAR THILAGAM DANA CINEMA 10:30,13:15,16:00,18:45,21:30,00:15 Cineco (20) 11.00 Am, 1.00, 3.00, 5.00, 7.00, 9.00, 11.00 (TAMIL/BIOGRAPHY/DRAMA ) Seef (I) From Friday 25Th:12.00, 6.00 Pm, 12.00 Mn for his role Keerthi Suresh, Dulquer Salmaan, Samantha Ruth Prabhu Al Hamra From Friday 25Th: 3.00 Pm A Quiet Place DANA CINEMA -

23.10.2000 30-Jun-21 Insurer: ROYAL
Registration No. 102 Date of Registration with the IRDA : 23.10.2000 Insurer: ROYAL SUNDARAM GENERAL INSURANCE CO. LIMITED Date: 30-Jun-21 S.No Agent Name IRDA URN License No AGCode District 1 Salesh Arora LICI2301088663 483320 AG000628 Delhi 2 KishorJoshi RSAI1409090261 1333470 AG000978 Delhi 3 Kailash Chandra Srivastava OKMI1803081925 319096 AG000753 Delhi 4 Guruprasad S Rao LICI1408088658 789116 AG000985 Pune 5 Abhijit Godbole RSAI1307090228 656692 AG000988 Pune 6 R.RAMESH BABU LICI1105073694 186541 AG001327 Pune 7 Bhadresh H Parekh MAXI3103085537 517485 AG001268 Mumbai 8 Menon R K MAXI2711073021 358144 AG001264 Mumbai 9 Voruganti Durga P Nath RSAI1901120003 9040972 AG001425 Vijayawada 10 Bindu J Dhabaria LICI2402098648 542670 AG001446 Kolkata 11 Swati B Mehta LICI0412072110 288237 AG001047 Mumbai 12 Anup Kumar Guha ICII1306088320 573759 AG001377 Mumbai 13 Abhishek Garg RSAI1803089966 355927 AG001537 Kolkata 14 Sandeep Karkhanis ABLI1107074977 280732 AG001342 Pune 15 G V Selvha Muthu Kumaar BSLI3103083027 530951 AG001655 Chennai 16 Abhay Bora HDFI3001090511 934478 AG001711 Pune 17 Aliasgar Z Sabuwala RSAI0406090207 347779 AG001722 Pune 18 S.Elango LICI2409070731 1758304 AG001693 Pune 19 George Anto Thomas LICI1010089658 838349 AG001797 Mumbai 20 BHATT SAJAL D RSAI0109090256 1267239 AG001812 Mumbai 21 GHAI PAVANNEET SINGH BSLI1810046758 287211 AG001814 Mumbai 22 SHAH KUNTAL L( Lalit Shah) RSAI2206069716 1267213 AG001791 Mumbai 23 Archana S. Kashettiwar ICII2302090592 1011846 AG001875 Mumbai 24 R. Mani RSAI1105090204 1240441 AG001903 -

Building Mental Health Research Capacity in India
2018 Building Mental Health Research Capacity in India Supported by the Fogarty International Center National Institutes of Health Department of Epidemiology College of Public Health and Health Professions and College of Medicine Compiled and Designed by Krishna Vaddiparti, PhD, MPE, MSW Assistant Professor Coordinator and Mentor, Fogarty Indo-US Training Program Coordinator MPH Epidemiology Program Department of Epidemiology College of Public Health & Health Professions & College of Medicine 2004 Mowry Road PO Box 100231, Gainesville, FL 32611 Phone: 352-273-5746, Fax: 352-273-5365 [email protected] epidemiology.phhp.ufl.edu 1 Dedicated to Theodore Reich, MD. (1938–2003) Dr. Reich was professor of psychiatry and genetics at Washington University School of Medicine and is still considered one of the most influential thinkers of modern psychiatric genetics. Dr. Reich was the founding PI of the Fogarty Indo-US Training Program and was a beloved mentor to many. 2 ICOHRTA FOGARTY INDO-US TRAINING PROGRAM ON BEHAVIORAL DISORDERS ACROSS THE LIFESPAN D43TW5811 2001—2011 Washington University School of Medicine Linda B Cottler, PhD, MPH, FACE Program Director Washington University St. Louis, MO, United States Sanjeev Jain, MD Major Foreign Collaborator National Institute of Mental Health and Neuro Sciences, Bangalore, India 3 Training Mentors India Vivek Benegal, MD Prabha Chandra, MD, MRC Psych Gururaj Gopalkrishna, MD PhD Sanjeev Jain, MD Pratima Murthy, MD Janardhan Reddy, MD Shoba Srinath, MD Mathew Varghese, MD United States Arpana Agrawal, -

DT Page 01 May 09.Indd
www.thepeninsulaqatar.com CAMPUS | 4 COMMUNITY | 5 ENTERTAINMENT | 11 PEC team enters NIA celebrates Working with Qatar Debate Indian harvest Ilayaraja fun: final festival Kamal Haasan MONDAY 9 MAY 2016 Email: [email protected] thepeninsulaqatar @peninsulaqatar @peninsula_qatar Some banks, tax agencies and tech companies are mak- ing the selfie an inte- gral step for people checking their bank accounts, shopping online and filing tax returns. SELFIES AS PASSWORDS P | 2-3 02 | MONDAY 9 MAY 2016 COVER STORY Your password could be written all over your face with selfie security that difficult to find out what someone looks like. “Everyone has your face,” says Alvaro Bedoya, the executive director of Georgetown Law’s Center on Privacy and Technology. “So it is a mode of authentication that is inherently public.” To overcome that risk, the companies are re- quiring selfies that are a little different than the ones you might see on Facebook. After finding the right angle, consumers are asked to move around to confirm that the cam- era is capturing a live person and not a photo. In the MasterCard and USAA programmes, users are told when to blink. Georgia’s tax pro- gramme will prompt people to position their faces a certain way and scan for motion. The photos are typically not the only safety measure, serving instead as the second or third method of authentication. USAA, for example, says that it checks not only the photo, but also for the device being By Jonnelle Marte For instance, MasterCard plans to roll out used to access the account.