Bkecciation, Alteration, and Mineralization at The
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Sand-Gravel Marine Deposits and Grain-Size Properties
GRAVEL ISSN 1678-5975 Novembro - 2005 Nº 3 59-70 Porto Alegre Sand-Gravel Marine Deposits and Grain-Size Properties L. R. Martins1,2 & E. G. Barboza2 1 COMAR- South West Atlantic Coastal and Marine Geology Group; 2 Centro de Estudos de Geologia Costeira e Oceânica – CECO/IG/UFRGS. RESUMO A plataforma continental Atlântica do Rio Grande do Sul e Uruguai foi utilizada como laboratório natural para testar as relações entre propriedades de tamanho de grão e ambiente sedimentar. A evolução Pleistoceno/Holoceno da região foi intensamente estudada através de um mapeamento detalhado, e de estudos sedimentológicos e estratigráficos, oferecendo, dessa forma, uma excelente oportunidade para esse tipo de trabalho. Acumulações de areia e cascalho, vinculadas a níveis de estabilização identificados da transgressão Holocênica, localizados nas isóbatas de 110-120 e 20-30 metros, fornecem elementos confiáveis relacionados com a fonte, transporte e nível de energia de deposição e podem ser utilizados como linhas de evidencias na interpretação ambiental. ABSTRACT The Atlantic Rio Grande do Sul (Brazil) and Uruguay inner continental shelf was used as a natural laboratory to test the relationship between grain-size properties and sedimentary environment. The Pleistocene/Holocene evolution of the region was intensively studied through detailed mapping, sedimentological and stratigraphic research thus offering an excellent opportunity of developing this type of work. Sand and gravel deposits linked with identified stillstands of the Holocene transgression located at 110-120 and 20-30 meters isobath provided elements related to the source, transport and depositional energy level and can be used as a tool for environmental interpretation. Keywords: marine deposits, grain-size, sand-gravel, Holocene. -

45. Sedimentary Facies and Depositional History of the Iberia Abyssal Plain1
Whitmarsh, R.B., Sawyer, D.S., Klaus, A., and Masson, D.G. (Eds.), 1996 Proceedings of the Ocean Drilling Program, Scientific Results, Vol. 149 45. SEDIMENTARY FACIES AND DEPOSITIONAL HISTORY OF THE IBERIA ABYSSAL PLAIN1 D. Milkert,2 B. Alonso,3 L. Liu,4 X. Zhao,5 M. Comas,6 and E. de Kaenel4 ABSTRACT During Leg 149, a transect of five sites (Sites 897 to 901) was cored across the rifted continental margin off the west coast of Portugal. Lithologic and seismostratigraphical studies, as well as paleomagnetic, calcareous nannofossil, foraminiferal, and dinocyst stratigraphic research, were completed. The depositional history of the Iberia Abyssal Plain is generally characterized by downslope transport of terrigenous sedi- ments, pelagic sedimentation, and contourite sediments. Sea-level changes and catastrophic events such as slope failure, trig- gered by earthquakes or oversteepening, are the main factors that have controlled the different sedimentary facies. We propose five stages for the evolution of the Iberia Abyssal Plain: (1) Upper Cretaceous and lower Tertiary gravitational flows, (2) Eocene pelagic sedimentation, (3) Oligocene and Miocene contourites, (4) a Miocene compressional phase, and (5) Pliocene and Pleistocene turbidite sedimentation. Major input of terrigenous turbidites on the Iberia Abyssal Plain began in the late Pliocene at 2.6 Ma. INTRODUCTION tured by both Mesozoic extension and Eocene compression (Pyrenean orogeny) (Boillot et al., 1979), and to a lesser extent by Miocene com- Leg 149 drilled a transect of sites (897 to 901) across the rifted mar- pression (Betic-Rif phase) (Mougenot et al., 1984). gin off Portugal over the ocean/continent transition in the Iberia Abys- Previous studies of the Cenozoic geology of the Iberian Margin sal Plain. -

Orientation, Sphericity and Roundness Evaluation of Particles Using Alternative 3D Representations
Orientation, Sphericity and Roundness Evaluation of Particles Using Alternative 3D Representations Irving Cruz-Mat´ıasand Dolors Ayala Department de Llenguatges i Sistemes Inform´atics, Universitat Polit´ecnicade Catalunya, Barcelona, Spain December 2013 Abstract Sphericity and roundness indices have been used mainly in geology to analyze the shape of particles. In this paper, geometric methods are proposed as an alternative to evaluate the orientation, sphericity and roundness indices of 3D objects. In contrast to previous works based on digital images, which use the voxel model, we represent the particles with the Extreme Vertices Model, a very concise representation for binary volumes. We define the orientation with three mutually orthogonal unit vectors. Then, some sphericity indices based on length measurement of the three representative axes of the particle can be computed. In addition, we propose a ray-casting-like approach to evaluate a 3D roundness index. This method provides roundness measurements that are highly correlated with those provided by the Krumbein's chart and other previous approach. Finally, as an example we apply the presented methods to analyze the sphericity and roundness of a real silica nano dataset. Contents 1 Introduction 2 2 Related Work 4 2.1 Orientation . 4 2.2 Sphericity and Roundness . 4 3 Representation Model 7 3.1 Extreme Vertices Model . 7 4 Sphericity and Roundness Evaluation 9 4.1 Oriented Bounding Box Computation . 9 4.2 Sphericity Computation . 11 4.3 Roundness Computation . 12 5 EVM-roundness -
Particle Shape
PARTICLE SHAPE Prepared By : Bhavuk Sharma Course : M.Sc. Geology Assistant Professor Semester – IV Department of Geology , Course Code : MGELEC-1 Patna University , Patna Unit : Unit - III [email protected] CONTENTS 1. Introduction 2. Definitions 3. Measuring Particle Shape 4. Particle Form 5. Particle Roundness 6. Surface Texture 7. Exercise 8. References. INTRODUCTION The shape of sedimentary particles is an important physical attribute that may provide information about the sedimentary history of a deposit or the hydrodynamic behavior of particles in a transporting medium. It is determined by the following factors : Orientation and Original shapes of Spacing of mineral grain in fractures in the source rocks bedrock Nature and Sediment burial Intensity of processes sediment transport Particle shape, however, is a complex function of lithology, particle size, the mode and duration of transport, the energy of the transporting medium, the nature and extent of post-depositional weathering, and the history of sediment transport and deposition. DEFINITIONS Particle Shape is defined by three related but different aspects of grains : Form , Roundness and Surface Texture Measuring PARTICLE SHAPE • Standardized numerical shape indices have been developed to facilitate shape analyses by mathematical or graphical methods. • Quantitative measures of shape can be made on two-dimensional images or projections of particles or on the three-dimensional shape of individual particles. • Two-dimensional particle shape measurements are particularly applicable when individual particles cannot be extracted from the rock matrix. • Three-dimensional analyses of individual irregularly shaped particles generally involve measuring the principal axes of a triaxial ellipsoid to approximate particle shape. • The two-dimensional particle shape is generally considered to be a function of attrition and weathering during transport whereas three-dimensional shape is more closely related to particle lithology. -

Identifying Sediment Transport Mechanisms from Grain Size-Shape Distributions
https://doi.org/10.5194/esurf-2019-58 Preprint. Discussion started: 25 November 2019 c Author(s) 2019. CC BY 4.0 License. Identifying sediment transport mechanisms from grain size-shape distributions Johannes Albert van Hateren1, Unze van Buuren1, Sebastiaan Martinus Arens2, Ronald Theodorus van Balen1,3, Maarten Arnoud Prins1 5 1Faculty of Science, Department of Earth Sciences, Vrije Universiteit, Amsterdam, 1081 HV, The Netherlands 2Bureau for Beach and Dune Research, Soest, The Netherlands 3TNO-Geological Survey of the Netherlands, Utrecht, 3584 CB, The Netherlands Correspondence to: Hans van Hateren ([email protected]) 10 Abstract. The way in which sediment is transported (creep, saltation, suspension), is traditionally interpreted from grain size distribution characteristics. However, the grain size range associated with transitions from one transport mode to the other is highly variable because it depends on the amount of transport energy available. In this study we present a novel methodology for determination of the sediment transport mode based on grain size and shape data from dynamic image analysis. The data are integrated into grain size-shape distributions and primary components are determined using end-member modelling. In 15 real-world datasets, primary components can be interpreted in terms of different transport mechanisms and/or sediment sources. Accuracy of the method is assessed using artificial datasets with known primary components that are mixed in known proportions. The results show that the proposed technique accurately identifies primary components with the exception of those primary components that only form minor contributions to the samples (highly mixed components). 20 The new method is also tested on sediment samples from an active aeolian system in the Dutch coastal dunes. -
Gravity Erosion
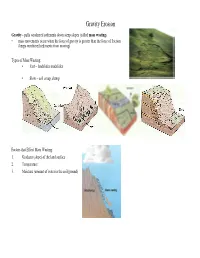
Gravity Erosion Gravity - pulls weathered sediments down steep slopes (called mass wasting ) • mass movements occur when the force of gravity is greater than the force of friction (keeps weathered sediments from moving) Types of Mass Wasting: • Fast – landslides mudslides • Slow – soil creep, slump Factors that Effect Mass Wasting: 1. Gradient (slope) of the land surface 2. Temperature 3. Moisture (amount of water in the soil/ground) Wind Erosion Wind - heavy winds can move sand, but rarely more than a meter above the ground and only where it is very dry • light winds can only move the smallest sediments • occurs in arid climates and coastlines where where loose sediments are available Deflation - process where winds blow away loose sediments, lowering the land surface Abrasion - winds blow sand against rocks and other objects causing them to be "sandblasted" Arches Hoodoos Water Erosion Streams - running water is the dominant form of erosion • the amount (volume) of water in a stream is called the stream's discharge Factor's affecting a stream's discharge: 1. Season - discharge greatest in the spring 2. Climate - greatest in humid climates 3. Ground/Soil - greatest when soil is saturated 4. Weather - increases after a period of precipitation Streams carry sediments by: 1. Suspension - carried within the water column 2. Bouncing/Rolling - larger particles along the stream bottom 3. In-solution - minerals dissolved in the water • as sediments move in the water, the hit rocks, the stream channel, and other sediments - this causes the sediments to become rounded in a process called abrasion As the velocity of a stream increases, its kinetic energy increases and the amount of erosion it does will increase Factors that Affect Stream Velocity: 1. -

Lab 4: Textures and Identification of Sedimentary Rocks
LAB 4: TEXTURES AND IDENTIFICATION OF SEDIMENTARY ROCKS OBJECTIVES 1) to become familiar with the properties important in recognizing and classifying sedimentary rocks 2) to become familiar with the textures characteristic of sedimentary rocks; 3) to become familiar with the mineralogy of common sedimentary rocks. INTRODUCTION Sedimentary rocks are rocks formed by deposition from a fluid (i.e., water, air, or ice). They are classified on the basis of their texture, grain size, and mineralogic composition. Characteristics of sedimentary rocks are described in Pellant p. 38-41and 44-45; Marshak, p. 176-186. Texture: Sedimentary rocks may have clastic (detrital) or non-clastic texture. Clastic sedimentary rocks are composed of grains, fragments of pre-existing rocks that have been packed together with spaces (pores) between grains. These pores may later be filled in with cementing materials such as silica or calcite deposited by groundwater moving through the sediment. Examples of clastic sedimentary rocks are sandstone and conglomerate. Some clastic sedimentary rocks (such as shale and mudstone) are fine enough that the individual grains cannot be distinguished. These fine-grained rocks are said to have an aphanitic texture. Non-clastic textures are found chiefly in rocks that have precipitated chemically from water (chemical sedimentary rocks), such as limestone, dolomite and chert. Other non-clastic sedimentary rocks include those formed by organisms (biochemical rocks), and those formed from organic material, such as coal. Rocks formed mainly from shell fragments are technically clastic rocks, but are commonly classed with the non-clastic ones because they too are chemical precipitates - except that organisms did the precipitating. -

Shape and Erosion of Pebbles
University of Pennsylvania ScholarlyCommons Department of Physics Papers Department of Physics 2-5-2007 Shape and Erosion of Pebbles Douglas Durian University of Pennsylvania, [email protected] H. Bideaud LDFC-CNRS UMR P. Duringer Institut de Géologie A. P. Schröder LDFC-CNRS UMR; Institut Charles Sadron C. M. Marques LDFC-CNRS UMR; Institut Charles Sadron Follow this and additional works at: https://repository.upenn.edu/physics_papers Part of the Physics Commons Recommended Citation Durian, D., Bideaud, H., Duringer, P., Schröder, A. P., & Marques, C. M. (2007). Shape and Erosion of Pebbles. Retrieved from https://repository.upenn.edu/physics_papers/158 Suggested Citation: D.J. Durian, H. Bideaud, P. Duringer, A.P. Schröder. amd C.M. Marques. (2007). Shape and erosion of pebbles. Phsyical Review E 75, 021301. © 2007 The American Physical Society http://dx.doi.org/10.1103/PhysRevE.75.021301 This paper is posted at ScholarlyCommons. https://repository.upenn.edu/physics_papers/158 For more information, please contact [email protected]. Shape and Erosion of Pebbles Abstract The shapes of flat pebbles may be characterized in terms of the statistical distribution of curvatures measured along their contours. We illustrate this method for clay pebbles eroded in a controlled laboratory apparatus, and also for naturally occurring rip-up clasts formed and eroded in the Mont St.- Michel bay. We find that the curvature distribution allows finer discrimination than traditional measures of aspect ratios. Furthermore, it connects to the microscopic action of erosion processes that are typically faster at protruding regions of high curvature. We discuss in detail how the curvature may be reliably deduced from digital photographs. -

Shape and Erosion of Pebbles Douglas Durian, H
Shape and erosion of pebbles Douglas Durian, H. Bideaud, P. Duringer, André Schröder, Carlos Marques To cite this version: Douglas Durian, H. Bideaud, P. Duringer, André Schröder, Carlos Marques. Shape and erosion of pebbles. Physical Review E : Statistical, Nonlinear, and Soft Matter Physics, American Physical Society, 2007, 75 (2), pp.021301. 10.1103/PhysRevE.75.021301. hal-00144239 HAL Id: hal-00144239 https://hal.archives-ouvertes.fr/hal-00144239 Submitted on 16 Jun 2007 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. The shape and erosion of pebbles D.J. Durian,1,2 H. Bideaud,2 P. Duringer,3 A. Schr¨oder,2 C.M. Marques2 1Department of Physics & Astronomy, University of Pennsylvania, Philadelphia, PA 19104-6396, USA 2LDFC-CNRS UMR 7506, 3 rue de l’Universit´e,67084 Strasbourg Cedex, France and 3CGS-CNRS UMR 7517, Institut de G´eologie, 1 rue Blessig, 67084 Strasbourg Cedex, France (Dated: November 28, 2006) The shapes of flat pebbles may be characterized in terms of the statistical distribution of curvatures measured along their contours. We illustrate this new method for clay pebbles eroded in a controlled laboratory apparatus, and also for naturally-occurring rip-up clasts formed and eroded in the Mont St.-Michel bay. -
An Introduction to Geology
An Introduction to Geology About this free course This free course is an adapted extract from the FutureLearn course The Earth in My Pocket: An Introduction to Geology. This version of the content may include video, images and interactive content that may not be optimised for your device. You can experience this free course as it was originally designed on OpenLearn, the home of free learning from The Open University – https://www.open.edu/openlearn/science-maths-technology/introduction-geology/content-section-over- view There you’ll also be able to track your progress via your activity record, which you can use to demonstrate your learning. Copyright © 2019 The Open University Intellectual property Unless otherwise stated, this resource is released under the terms of the Creative Commons Licence v4.0 http://creativecommons.org/licenses/by-nc-sa/4.0/deed.en_GB. Within that The Open University interprets this licence in the following way: www.open.edu/openlearn/about-openlearn/frequently-asked-questions-on-openlearn. Copyright and rights falling outside the terms of the Creative Commons Licence are retained or controlled by The Open University. Please read the full text before using any of the content. We believe the primary barrier to accessing high-quality educational experiences is cost, which is why we aim to publish as much free content as possible under an open licence. If it proves difficult to release content under our preferred Creative Commons licence (e.g. because we can’t afford or gain the clearances or find suitable alternatives), we will still release the materials for free under a personal end- user licence. -
Cross-Shore Distribution of Sediment Texture Under Breaking Waves Along Low-Wave-Energy Coasts Ping Wang Louisiana State University, [email protected]
University of South Florida Masthead Logo Scholar Commons Geology Faculty Publications Geology 5-1998 Cross-Shore Distribution of Sediment Texture Under Breaking Waves Along Low-Wave-Energy Coasts Ping Wang Louisiana State University, [email protected] Richard A. Davis Jr. University of South Florida Nicholas C. Kraus U.S. Army Engineer Research and Development Center Follow this and additional works at: https://scholarcommons.usf.edu/gly_facpub Part of the Geology Commons Scholar Commons Citation Wang, Ping; Davis, Richard A. Jr.; and Kraus, Nicholas C., "Cross-Shore Distribution of Sediment Texture Under Breaking Waves Along Low-Wave-Energy Coasts" (1998). Geology Faculty Publications. 177. https://scholarcommons.usf.edu/gly_facpub/177 This Article is brought to you for free and open access by the Geology at Scholar Commons. It has been accepted for inclusion in Geology Faculty Publications by an authorized administrator of Scholar Commons. For more information, please contact [email protected]. CROSS-SHORE DISTRIBUTION OF SEDIMENT TEXTURE UNDER BREAKING WAVES ALONG LOW-WAVE-ENERGY COASTS PING WANG1, RICHARD A. DAVIS, JR.2, AND NICHOLAS C. KRAUS3 1 Coastal Studies Institute, Louisiana State University, Baton Rouge, Louisiana 70803, U.S.A. 2 Department of Geology, University of South Florida, Tampa, Florida 33620, U.S.A. 3 U.S. Army Engineer Waterways Experiment Station, Coastal and Hydraulics Laboratory, 3909 Halls Ferry Road, Vicksburg, Mississippi 39180, U.S.A. ABSTRACT: Sediment samples were collected with streamer traps at tative average values, and the amount of sediment collected, usually less different elevations in the water column and across the surf zone. than 1 g, may not be suf®cient to obtain a reliable grain-size distribution Beach pro®les and breaking waves were measured together with the for poorly sorted sediment. -
Beach Gravels As a Potential Lithostatistical Indicator of Marine Coastal Dynamics: the Pogorzelica–Dziwnów (Western Pomerania, Baltic Sea, Poland) Case Study
geosciences Article Beach Gravels as a Potential Lithostatistical Indicator of Marine Coastal Dynamics: The Pogorzelica–Dziwnów (Western Pomerania, Baltic Sea, Poland) Case Study Cyprian Seul 1,*, Roman Bednarek 1, Tomasz Kozłowski 1 and Łukasz Maci ˛ag 2 1 Faculty of Civil and Environmental Engineering, West Pomeranian University of Technology in Szczecin, 70-310 Piastów 50, Poland; [email protected] (R.B.); [email protected] (T.K.) 2 Institute of Marine and Environmental Sciences, University of Szczecin, Mickiewicza 18, 70-383 Szczecin, Poland; [email protected] * Correspondence: [email protected] Received: 12 May 2020; Accepted: 14 September 2020; Published: 16 September 2020 Abstract: The petrographic composition and grain shape variability of beach gravels in the Pogorzelica–Dziwnów coast section (363.0 to 391.4 km of coastline), southern Baltic Sea, Poland were analyzed herein to characterize the lithodynamics and trends of seashore development. Gravels were sampled at 0.25 km intervals, in the midpart of the berm, following an early-autumn wave storm and before beach nourishment. Individual variations in petrographic groups along the shore were investigated. Gravel data were compared and related to coastal morpholithodynamics, seashore infrastructure, and geology of the study area. The contribution of crystalline rock gravels (igneous and metamorphic) was observed to increase along all coast sections, whereas the amount of less resistant components (limestones, sandstones, and shales) usually declined. This effect is explained by the greater wave crushing resistance of igneous and metamorphic components, compared with sedimentary components. Similarly, the gravel grain shape (mainly elongation or flattening) was observed to change, depending on resistance to mechanical destruction, or due to the increased chemical weathering in mainly the limestones, marbles, and sandstones.