GRAPE MATURITY Section 3. Ph and Acidity Ph and Potassium (K ) for A
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Wine Spectator
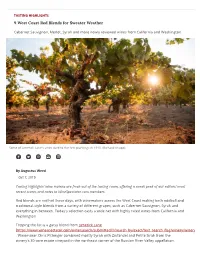
TASTING HIGHLIGHTS 9 West Coast Red Blends for Sweater Weather Cabernet Sauvignon, Merlot, Syrah and more newly reviewed wines from California and Washington Some of Limerick Lane's vines date to the !rst plantings in 1910. (Richard Knapp) By Augustus Weed Oct 7, 2019 Tasting Highlights' wine reviews are fresh out of the tasting room, o!ering a sneak peek of our editors' most recent scores and notes to WineSpectator.com members. Red blends are red hot these days, with winemakers across the West Coast making both oddball and traditional-style blends from a variety of di"erent grapes, such as Cabernet Sauvignon, Syrah and everything in between. Today's selection casts a wide net with highly rated wines from California and Washington. Topping the list is a gutsy blend from Limerick Lane [https://www.winespectator.com/wine/search/submitted/Y/search_by/exact/text_search_#ag/winery/winery/Limerick+Lane] . Winemaker Chris Pittenger combined mostly Syrah with Zinfandel and Petite Sirah from the winery's 30-acre estate vineyard in the northeast corner of the Russian River Valley appellation. Alexana [https://www.winespectator.com/wine/search/submitted/Y/search_by/exact/text_search_#ag/winery/winery/Alexana] winemaker Bryan Weil looked farther north to the Columbia Valley in Washington for the supple Gran Rouge. It's a Southern Rhône–inspired blend of Grenache, Syrah and Mourvèdre that shows how well these grapes complement each other. Eric Kent [https://www.winespectator.com/wine/search/submitted/Y/search_by/exact/text_search_#ag/winery/winery/Eric+Kent] made one of the best values here, using grapes from Mendocino County. -

View Our Hand Selected Wine List
RED BOTTLES Cabernet Sauvignon 14 Hands (Washington State) Alexander Valley Vineyards (Sonoma) Bevel (Paso Robles) Foxglove (Paso Robles) Ferrari Carano (Alexander) Duckhorn Decoy (Napa) Whitehall Lane (Napa) Jordan (Alexander) Silver Oak (Alexander) Joseph Phelps (Napa) Cakebread (Napa) Merlot Velvet Devil (Washington State) Monkey Business (North Coast) Shafer (Napa) Duckhorn (Napa) Pinot Noir Au Bon Climat (Santa Barbara) Sean Minor (Carneros) Mark West (California) Talbott ”Logan” Sleepy Hollow Vineyards (Santa Lucia Highlands) Patz & Hall (Sonoma Coast) (Pictures may not represent current vintages) BLENDS Red Blend, Sean Minor (Napa) (Merlot, Petit Verdot, Zinfandel, Petite Sirah, Syrah, Malbec) Cinnabar, Mercury Rising (California) (Cabernet Sauvignon, Merlot, Cabernet Franc, Petite Verdot, Malbec) Abstract, Orin Swift Cellars (Napa) (Grenache, Petite Sirah, Syrah) Paraduxx, Duckhorn (Napa) (Zinfandel, Cabernet Sauvignon) The Prisoner, Prisoner Wine Co. (Napa) (Zinfandel, Cabernet Sauvignon, Syrah, Petite Syrah, Grenache) Papillon, Orin Swift (Napa) (Cabernet Sauvignon, Merlot, Cabernet Franc, Malbec, Petit Verdot) Zinfandel Il Cuore, (Mendocino County) 7 Deadly Zins, (Lodi) Turley, Juvenile (Napa) Syrah/Shiraz Qupe, (Central Coast) Shiraz, Woop Woop, (Australia) (Pictures may not represent current vintages) French Cotes du Rhone, Domaine Lafond “Roc-Epine” LA 50/50 (Minervois) (Old Vine Grenache, Carigan, Cinsault, Syrah) Chateau La Grace Dieux, Grand Cru (St. Emilion) Chateauneuf du Pape, Sixtine Italian Barbera D’Alba (Rocche -

Wine List Champagne & Sparkling Wines Glass Bottle Jaume Serra “Cristalino” Brut Cava, Spain, NV $10 $38
Wine List Champagne & Sparkling Wines Glass Bottle Jaume Serra “Cristalino” Brut Cava, Spain, NV $10 $38 Mionetto, Processco Brut, Italy (187 ml. Split) $16 G.H. Mumm “Cordon Rouge” Brut Champagne, Reims, France, NV $90 Veuve Cliquot “Yellow Label” Brut Champagne, Reims, France, NV (375 ml. half bottle) $46 White Wines The Beachcomber, Chardonnay, California, 2018 $10 $38 Butter, Chardonnay, California, 2018 $11 $42 La Crema, Chardonnay, Sonoma Coast, 2018 $12 $46 ZD, Chardonnay, California, 2017 $11 $42 Boathouse, Chardonnay, New Zealand, 2019 $58 Rombauer Vineyard, Chardonnay, Carneros, 2019 $19 $74 Ruffino Lumina, Pinot Grigio, Delle Venezie , 2019 $10 $38 Coppola, Sofia, Riesling, Monterey County, 2016 $12 $46 Matanzas Creek, Sauvignon Blanc, Sonoma County, 2018 $11 $42 Kim Crawford, Sauvignon Blanc, Marlborough, New Zealand, 2019 $13 $50 Rosé Wine Tolosa, Edna Valley, 2019 $10 $38 Boathouse, New Zealand, 2019 $46 Red Wines Glass Bottle Summerland, Pinot Noir, Santa Barbara County, 2017 $13 $50 Ken Brown, Pinot Noir, Santa Barbara County, 2017 $14 $54 The Federalist, Zinfandel, Lodi, 2017 $11 $42 Rombauer Vineyards Zinfandel, California, 2017 $60 Ridge “Lytton Springs”, Zinfandel, Deer Creek Valley, 2017 $80 Markham, Merlot, Napa Valley, 2017 $12 $46 Rombauer Vineyards, Merlot, 2017 $80 Duckhorn, Merlot, Napa Valley, 2015 $95 The Beachcomber, Cabernet Sauvignon, California, 2017 $12 $46 Daou, Cabernet Sauvignon, Paso Robles, 2018 $14 $54 Educated Guess, Cabernet Sauvignon, Napa Valley, 2017 $15 $58 Justin Vineyards, Cabernet Sauvignon, -
Wine Selection Wine Selection Wine Selection Artisan Mixed Drinks Non-Alcoholic Beverages Dessert
Wine Selection Wine Selection Wine Selection Artisan mixed drinks Non-Alcoholic Beverages Dessert Winemaker’s Whiskey Flight 16 Alcohol-Free Mudslide 7 Banana Split 13 Made with superior quality fruit and gluten Rose + Blush glass bottle Sparkling glass bottle Selected flight of rye, bourbon and whiskey Milk, chocolate syrup, coffee, vanilla Vanilla semifreddo, brûléed banana, candied walnuts, free, LaBelle Wines are free of additives or Premium Selection 25 fudge, Chantilly, luxardo cherry, sprinkles Rosé 11 34 Shimmer 13 45 Ginless Tonic 8 chemicals, allowing the fruit to express Dry, classic, fruity, elegant Seyval Blanc and Dry Riesling blend, delicate, crisp, floral House-made juniper syrup, fresh lime, Flourless Chocolate Torte 11 naturally. I hope you enjoy drinking LaBelle Cranberry Cosmopolitan 12 muddled cucumber, tonic 13 45 Cold brew cream, smoked salt caramel Wine as much as I love making it for you.” Corazon Rosé 11 34 Tempest Cranberry wine, fresh lime juice, triple sec, The Off-dry blend, citrus, fruity Red Raspberry, Seyval Blanc and Baco Noir blend Alcohol-Free Strawberry Margarita 7 – Amy LaBelle, Winemaker Winemaker’s Kitchen Cranberry Sugar rim House-made sour mix, muddled Blueberry Cobbler 12 Cranberry 11 34 premium wines strawberries, agave nectar, Sprite Cornmeal biscuit, lemon ice cream Wine Flights Sweet-tart, crisp, fresh, New England cranberries Raspberry Lemon Drop 12 Raspberry Lime Rickey 8 from around the world bottle Red Raspberry wine, vodka, triple sec, fresh lemon juice 3 (2 oz pours) House-made juniper -

Winemaking Step by Step EQUIPMENT RED WINE PROCEDURES Crush (Break the Skins) and De-Stem the Grapes
Winemaking Step by Step EQUIPMENT RED WINE PROCEDURES Crush (break the skins) and de-stem the grapes. For most grape 1 varieties, about 90% of the larger stems should be removed. For most beginners, the hardest thing about making wine is simply figuring out, in advance, what Test for total acidity following the instructions in your acid testing equipment is going to be needed. This list 2 kit. If the acidity is less than .6%, add enough tartaric acid to bring it should set most of these fears to rest. to that level. If you have a pH meter, also test the pH. You will need the following: Test for sugar with your hydrometer. 3 Correct any deficiencies yb adding enough sugar to bring the reading up to at least 22° Brix or add water to bring the sugar down to a range 1. Siphon Hose and Racking Tube between 22° and 26° Brix. 2. Hydrometer and Test Jar 3. Acid Testing Kit When these tests and corrections have been completed, the must 4 should be sulfited. Estimating that you will get roughly one gallon of 4. SulfiteTest juice yield for every 16 lbs. of grapes, calculate the anticipated amount 5. Crusher or Crusher/Destemmer of juice. Using this estimate, add enough sulfite to give you a sulfur 6. Press or Pressing Bag dioxide (SO2) level between 50 and 130 parts per million (ppm). The 7. Thermometer amount needed will depend on the condition of the grapes, with 8. Scoop with Handle moldy grapes getting the most concentrated dose. Extremely clean 9. -

Wines by the Glass White Wine by the Bottle Red Wine By
WINES BY THE GLASS RED WINE BY THE BOTTLE CHAMPAGNE & SPARKLING GLASS BOTTLE CABERNET SAUVIGNON Prosecco, Giuliana 12 48 306 Andrew Will ‘Black Label’, 2016, Washington 76 Cremant de Bordeaux, Calvet, Brut Rose 13 52 308 Cakebread, 2017, Napa Valley 165 Champagne, Piper Heidsieck ‘1785’. Brut 20 80 310 Caymus, 2018, Napa Valley 175 314 Chateau Montelena, 2017, Napa Valley 125 WHITE WINE & ROSE 318 Chimney Rock ‘Tomahawk Vineyard’, 2013, Stag’s Leap Rose. Chapoutier ‘Belleruche’, 2018, Rhone, FR 12 48 District 218 Pinot Grigio, Terlato, 2018, Friuli, IT 14 56 324 Feather, 2015, Columbia Valley, WA 145 Riesling, The Seeker, 2018, Mosel, GER 11 44 342 Kate Arnold, 2017, Columbia Valley, WA 58 Sauvignon Blanc, Honig, 2018, Napa Valley 12 48 352 Newton ‘Unfiltered’, 2016, Napa Valley 106 Chenin Blanc, Graziano, 2016, Mendocino 11 44 354 Nickel & Nickel ‘CC Ranch’, 2017, Yountville 196 Chardonnay, Albert Bichot, 2016, Burgundy, FR 16 64 362 Pine Ridge, 2016, Napa Valley 82 Chardonnay, Talbott ‘Kali Hart’, 2017, Monterey 14 56 364 Sequoia Grove, 2016, Napa Valley 99 RED WINE 366 Silver Oak, 2015, Alexander Valley 165 Pinot Noir, Cloudline, 2018, Willamette, WA 13 52 376 Stag’s Leap Wine Cellars ‘Artemis’, 2017, Napa Valley 135 Barbera d’Asti, Pico Maccario ‘Lavignone’, 2017, PROPRIETARY BLENDS Piedmont, IT 11 44 400 Andrew Will ‘Sorella’ Champoux Vineyards, 2013, Sangiovese, Il Poggione Rosso di Montalcino, 2017, HHH, WA 165 Tuscany, IT 16 64 408 Cain ‘Cain Five’, 2007, Spring Mountain District 275 Zinfandel, Federalist, 2016, Lodi 12 48 412 Delille ‘D2’, 2015, Columbia Valley, WA 96 Syrah Blend, E. -

Factors Affecting Wine Texture, Taste, Clarity, Stability and Production Efficiency
Factors affecting wine texture, taste, clarity, stability and production efficiency FINAL REPORT to AUSTRALIAN GRAPE AND WINE AUTHORITY Project Number: AWRI 3.1.4 Principal Investigator: Dr Paul Smith Research Organisation: The Australian Wine Research Institute Date: 22 September 2017 Project 3.1.4 – Factors affecting wine texture, taste, clarity, stability and production efficiency Abstract Wine texture is considered a major product differentiator both for wine style and value in the marketplace. In addition, clarity and colour stability (absence of haze development and the retention of colour) are generally considered to be pre-requisites to market success. Achieving the optimum levels for each of these parameters is often done at significant cost using current technology and does not always ensure the wine will meet its full potential. The ability to modulate these characteristics of wine while retaining the ability to economically process the wine to ensure microbial stability and stylistic integrity is a significant challenge for the wine industry. This project focused on the key compositional drivers behind texture, bitterness, clarity, stability (protein and colour) and wine filterability and developed strategies to modulate them in a production-based environment. Specifically, it covered molecular drivers of taste and texture, ‘smart’ surfaces for efficient production, predicting haze formation, understanding and preventing wine haze, effects of filtering red wines, solids management effects on white wine style and composition, impact of winemaking methods on wine macromolecules and texture, colour development/management and a practical method to determine extractable grape colour and tannin. Executive summary Wine texture is considered a major product differentiator both for wine style and value in the marketplace. -

Red Wine Varietal Characters
Red Wine Varietal Characters Pinot Noir Cabernet Shiraz Zinfandel Cabermet Sauvignon Franc Stalky Tomato leaf Black olive Herbal Tarragon Sappy Dusty White pepper Tomato Capsicum Pickle Asparagus Black pepper Pepper Dusty Rhubarb Capsicum Spice Spice Cherry Cranberry Green bean Raspberry Raspberry Blackberry Strawberry Leafy Redcurrant Cherry Blackcurrant Raspberry Herbal Cherry Loganberry Plum Cherry Seaweed Mulberry Blackberry Violet Blackberry Black olive Blackberry Blackcurrant Musk Plum Cherry Briar Briar Perfumed Violet Blackberry Plum Plum Mint Rose petal Blackcurrant Jammy Fruitcake Mineral Gamey Mulberry Menthol Walnut Pencil shaves Roast lamb Bramble Eucalyptus Cola Tobacco Barnyard Plum Aniseed Raisin Bacon fat Mint Licorice Earthy Gamay Earthy Menthol Gamey Tar Beetroot Eucalyptus Grilled meat Strawberry Tree bark Aniseed Salami Merlot Raspberry Forest floor Violet Earthy Redcurrant Moss Fruitcake Chocolate Sappy Boiled sweets Fungal Beetroot Leather Black olive Cherry Truffle Prune Soy Sage Banana Cola Tea leaf Tar Herbal Iris Tar Tobacco Mint Peonies Prune Grenache Strawberry Spice Tempranillo Raspberry Nebbiolo Pepper Cherry Sangiovese Herbal Spice Mulberry Green tea Raspberry Raspberry Bramble Dried rose Rhubarb Cherry Confectionery Blackberry Violet Caper Blackberry Bubblegum Blackcurrant Camphor Raspberry Damson Cherry Plum Cherry Sour cherry Bramble Blackberry Violets Plum Cherry stone Plum Briar Perfumed Aniseed Plum Balsamic Plum Anise Truffle Perfumed Earthy Orange peel Earthy Chestnut Spice Spice Gamey Beetroot Mocha Gamey Cold tea Meaty Fruitcake Tobacco Tobacco Tobacco Earthy Spice Tar Farmyard Brown sugar Prune Tobacco Burnt toffee © 2002 Recognose Pty Ltd www.aromadictionary.com. -

Cabernet Sauvignon, Merlot, Or Other Red Wine Grape 1 Gallon Recipe
Cabernet Sauvignon, Merlot, or other Red Wine Grape 1 gallon recipe Ingredients: 15 lbs Red Wine grapes 234 SE 12th Ave, Portland, OR 1 Campden tablet (503) 232-8793 www.fhsteinbart.com ½ tsp. Yeast Nutrient ½ tsp. Pectic Enzyme (to release color from skins) 1 packet Red Wine Yeast (Lalvin RC212 or seasonal Red Wine yeast is recommended) Titratable Acidity test kit pH meter or pH test papers 2 gallon primary fermentation bucket 1 gallon glass jug Procedure: Day 1 If growing or picking fruit, you will want to test Titratable Acidity, pH, and ° brix (sugar content) in your grapes before harvest. You want your TA (Titratable Acidity) to be around 6 grams/Liter, pH to be 3.3 to 3.6 and sugar to be a minimum of 22° Brix (or a specific gravity of 1.090). If you are buying pre- picked grapes, test the juice to see where your readings are and to see if you will need to adjust anything later on. Sanitize all equipment (everything that will touch your wine!) Crush and de-stem fruit into primary fermenter (2 gal. bucket) Crush 1 campden tablet and add to must (crushed fruit and juice), along with yeast nutrient, and pectic enzyme. Cover with a loose fitting lid, and let sit for 24 hours. Day 2 After 24 hrs. add yeast and let keep at a constant temperature between 65° F and 85° F. (The cooler temperatures will give you a fruit forward wine while the warm temperatures will give you an earthy “old world” style wine) Day 3 Fermentation will start and the mixture will bubble vigorously. -

A Beginner's GUIDE to Wine Making
A Beginner's GUIDE to Wine Making 1. Choose if you want to make wine from juice that you would buy, or if you are going to use your own grapes or fruit. 2. It will be easier to make wine form juice that you would purchase from a winery or other reputable juice producers. 3. DO NOT make wine from the Welch's Grape concentrate that you buy in stores. 4. Making wine from grapes or other fruit will be much harder and take longer, you will also need more equipment to do so. 5. Once you decide which you will do, and the recipe you wish to follow, you are ready to buy the materials that you will need. Making Wine from Juice 1. Put the juice into a clean carboy, add .5 grams per gallon of Potassium Metabisulfite. 2. Check your sugar level with the hydrometer. You should be somewhere around 21 Brix or Balling. If not, add sugar to the wine until your hydrometer reads 21 Brix. 3. If you are going to change the acid of a wine, now is the time to do this. Adding either Potassium Bicarbonate or Calcium Carbonate is used tp drop acid in wine. Where as Acid Blend or Citric Acid is added to raise acid in your wine. (If you buy juice, most of this should already be adjusted for you so this stem may be unneccessary) 4. Add Pectic Enzyme to juice according to package for amounts. 5. Add Yeast Nutrient to juice in reccomended amount. 6. Take the Yeast and get water (about 1/2 a cup per 5 gallon carboy) at about 100 degrees Farenhiet, mix the Yeast into the water along with 1/2 the package of Yeast Starter. -

Bee & Bramble: a Land of Mead and Honey
Reap&Eat Bee & Bramble: A land of mead and honey by Frances Nevill he rolling mountains of western North Carolina yield Ta diverse mixture of flora and fauna. The beekeeper benefits from the rich nectar flows that result in a medley of honeys available only in the Appalachians (Sourwood, we’re talking about you!). The making of mead, a wine crafted from fermented honey, is one of the many ways honey can be enjoyed and one “mazer” (the official term for a mead maker), Ivar Schloz of Bee & Bramble Meadery in Fairview, is aiming to change old world perceptions of mead wine. “I was a red wine and beer drinker, but I often got headaches the next morning,” says Schloz. “I tried some mead and really took a liking to it and noticed that… I wasn’t left with a headache the next morning.” Having decades of home brewing experience, Schloz tried his hand at mead making. After sharing it amongst friends while at music festivals, he started getting requests to purchase bottles of his homemade concoction. “People would tell me that they had never tried mead, or if they had, they felt it was too sweet or too mossy tasting—what I call the barnyard flavor,” he recalls. “I aimed for a dry, almost white wine quality, and that is where I started to notice that mead, particularly my recipe, could really be enjoyed by the wine drinker who enjoys a glass or two (or three) of wine a day.” When the economy downturn hit, Schloz, a metallurgical engineer by trade, found himself out of a job and decided to turn to his hobby of mead making into a potential full- 48 | Plough to Pantry | Winter 2016 time business. -

Jack Keller's Requested Recipes
Jack Keller began sharing his winemaking insight with the world in 1994 via “The Winemaking Home Page.” Jack was thrilled to gift, free to all, everything he knew about winemaking techniques, insights, and his award-winning recipes. He was a wine judge, writer, blogger, and champion of homemade wines and meads. Sadly, the winemaking community lost Jack on September 13, 2020. A dedicated group of homebrewers scoured the web archives of Jack’s work to compile this book of Jack’s recipes, hoping to preserve them for generations to come. Please enjoy this volume, and in Jack’s honor, pass it on. JACK KELLER’S • Zinfandel Wine 40 • Pear Wine 41-42 REQUESTED RECIPES • Star Fruit Wine 43 • Maraschino-Chocolate Sweet Mead 6 • Berlandieri Grape Wine 44 • Mimosa Flower Wine 7-8 • Apple Juice Wine 45 • Blanc Du Bois White Wine 9-10 • Pomegranate Wine 46 • Cranberry-Raspberry Social Wine 11 • Hibiscus Flower Wine 47 • Jam & Fruit Spread Wine 12 • Black Spanish Wine 48 • Jelly Wine 13 • Capsicumel 49-51 • Honeydew Melon Wine 14 • Apple & Cranberry Wine 52 • Mixed Berry Wine 15 • Chambourcin Wine 53 • Mincemeat Wine 16 • Blackberry Port Wine 54 • Highbush Blueberry Wine 17-18 • Edelweiss Grape Wine 55 • Burnet Flower Wine 19 • Niagara Grape Wine 56 • Locust Blossom Wine 20 • Black Currant (Dried) Wine 57 • Orange Blossom Wine 21-22 • Zucchini Wine 58 • Gorse Wine 23 • Custard Apple Wine 59 • Mint Jelly Wine 24 • Cooking Pear Wine 60-61 • Calendula Wine 25 • Gewürztraminer 62 • Day Lily Wine 26 • Vermouth 63 • Black Tea Wine 27 • Praline Dessert Wine