The Bewildering Taxonomy of Genus Ranunculus with Particular Reference to Kashmir Himalaya
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
ACHIEVEMENTS) (Since Ph.D
RESEARCH AND ANNUAL ACTION PLAN PROJECTS (ACHIEVEMENTS) (Since Ph.D. –––Till Date) Dr. S.K. Srivastava Scientist E & HOO Botanical Survey of India Northern Regional Centre Dehradun Project Name /Work Place Position Held Duration Flora of India Project (BSI Research Scholar) Taxonomic Revision of Indian Oleaceae JRF/SRF 1979 - 1983 (10 genera, 94 taxa) National Botanical Research Institute, Lucknow Ph.D. Thesis submitted to University of Lucknow. Ms. submitted to BSI for Flora of India. NEC Orchid Project Multiplication and Preserve. of Orchids of N.E. India SRF 1984 (1 yr.) Botanical Survey of India, Shillong Man and Biosphere Project Ecological Impact of Beas -Sutlej Link Project in H.P . Res. Assoc. 1984-1986 Botanical Survey of India, Dehradun Biological Screening of wild plant species Survey and collection of Wild Plants for Biological Res. Assoc. 1986-1989 Screening from Arunachal Pradesh Central Drug Research Institute JOINED BOTANICAL SURVEY OF INDIA IN 1989 Botanical Survey of India, Port Blair Position held Duration Project Completed Individually 1. Survey, exploration of Great Nicobar Islands ; Botanist 1989-1994 Writing of Flora of Andaman/Nicobar Islands 2. State Flora i. Flora of Andaman & Nicobar Islands Vol. II (Manuscript Submitted) ii. Flora of Andaman & Nicobar Islands Vol. III (Manuscript Submitted) [Contributed Taxonomic Treatment of ca 170 species] iii. Flora of Kerala State Vol. 1 (Family: Bombacaceae) Published with K. Vivekananthan) Botanical Survey of India, Allahabad (Botanist) (1994 -2000) 1. Flora of India (Individual Project) Revision of Genus Ischaemum L. (Tribe: Andropogoneae) Poaceae (55 spp.-Published with Dr. V. J. Nair) 2. Protected Area (Individual Project) Flora of Bandhavgarh National Park, Madhya Pradesh (529 spp; Project completed/Published under Tiger Reserve of India) 3. -
MASTER-STAFF Query
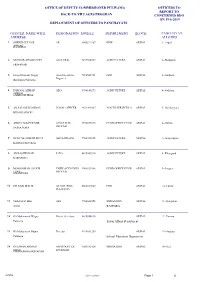
OFFICE OF DEPUTY COMMISSIONER PULWAMA OFFICERS TO REPORT TO BACK-TO-VILLAGES PROGRAM CONCERNED BDO ON 19-6-2019 DEPLOYMENT OF OFFICERS TO PANCHAYATS OFFICER NAME WITH DESIGNATION MOBILE DEPARTMENT BLOCK PANCHAYAT ADDRESS ALLOTED 1 SHEIKH GULZAR AE 9906822287 REWARIPAL 1--Aripal AHMAD KANDIZAL 2 MUNEER AHMAD SOFI AA(I) TRAL 9419064569 AGRICULTUREARIPAL 2--Batagund SRINAGAR 3 Javeed Hussain Wagay Asstt Executvive 9596565731 PDDARIPAL 3--Gudpora Engineer Barthipora Pulwama 4 FAROOQ AHMAD AEO 9596145273 AGRICULTUREARIPAL 4--Gulistan SHEIKH SAIMOOH TRAL 5 AB SALAM KHANDAY ZONAL OFFICER 9622490387 YOUTH SERVICES AARIPAL 5--Gulshanpora BHAN KAIMOH 6 ABDUL MAJEED MIR ACCOUNTS 9596033510 FUND OFFICE PULWARIPAL 6--Gutroo OFFICER ZAINA PORA 7 IMTIYAZ AHMAD BHAT AEO LORGAM 9906703035 AGRICULTUREARIPAL 7--Jawaherpora KAHNGUND TRAL 8 AB RASHID DAR JAEO 8825089926 AGRICULTUREARIPAL 8--Khangund KAKAPORA 9 MOHAMMAD AYOUB CHIEF ACCOUNTS 9906820386 FUND OFFICE PULWARIPAL 9--Lurgam LONE OFFICER ANANTNAG 10 GH NABI MALIK AE STD (PDD) 8803019822 PDDARIPAL 10--Lurow PULWAMA 11 Mohd Shafi Bhat AEE 9906608553 IRRIGATIONARIPAL 11--Nargistan Amlar RAJPORA 12 Gh Mohammad Wagay Private Secretary 9419054810 ARIPAL 12--Panzoo Pulwama Tribal Affairs Department 13 Gh Mohammad Hajam Director 9419641255 ARIPAL 13--Satoora Pulwama School Education Department 14 GULSHAN AHMAD ASSISTANT EX 9086312924 IRRIGATIONARIPAL 14--Seer SHAH ENGINEER CHANAPORA SRINAGAR ARIPAL 100 - reserve Page 1 of OFFICE OF DEPUTY COMMISSIONER PULWAMA OFFICERS TO REPORT TO BACK-TO-VILLAGES PROGRAM -

Greater Flamingo Phoenicopterus Roseus, Siberian Rubythroat Calliope
Correspondence 193 Four additions to the avifauna of Himachal Pradesh: plumage almost disappeared and they became all white, except Greater Flamingo Phoenicopterus roseus, Siberian for the flight feathers and bare parts. There was a hint of pink on Rubythroat Calliope calliope, Rufous Woodpecker their mantles and backs. The last sighting of these two individuals Micropternus brachyurus, and Great Hornbill was on 09 April 2017, by CA [203]. There was no further sighting Buceros bicornis of this species in subsequent winters. Himachal Pradesh is rich in avifauna. More than half of the total species of birds found in India have been reported from the state (Praveen et al. 2020; Dhadwal 2019). CA has been extensively exploring Pong Lake (also known as Maharana Pratap Sagar), a designated Ramsar Site. It is a large man-made reservoir, on the Beas River in Kangra District, with an area of 156.62 sq. km and follows an annual cycle of filling-up in the monsoons, and gradually draining off thereafter (Abhinav et al. 2018). VS and HC frequently explore Colonel Sher Jung National Park, and locations around Renuka Ji and Paonta Sahib in Sirmaur District. Colonel Sher Jung National Park (27.88 sq. km) was previously known as Simbalbara National Park, and is located in the lower Shivalik region of Sirmaur District in southern Himachal Pradesh. It comprises moist Sal Shorea robusta forests and northern dry Both: C. Abhinav mixed deciduous forests (Abhinav et al. 2019). We report here four birds that were first recorded by us in Himachal Pradesh, in chronological order. 203. Greater Flamingo at Nagrota Surian, Pong Lake, on 09 April 2017. -

The Genus Ranunculus : a Phytochemical and Ethnopharmacological Review
AAAcccaaadddeeemmmiiiccc SSSccciiieeennnccceeesss International Journal of Pharmacy and Pharmaceutical Sciences ISSN- 0975-1491 Vol 4, Suppl 5, 2012 Review Article THE GENUS RANUNCULUS : A PHYTOCHEMICAL AND ETHNOPHARMACOLOGICAL REVIEW M SHAHZAD ASLAM*, BASHIR A. CHOUDHARY, M UZAIR, A SUBHAN IJAZ Department of pharmacy, Bahauddin Zakariya University, Multan, Pakistan. Email: [email protected] Received: 12 july 2012, Revised and Accep17 agu 2012 ABSTRACT The genus Ranunculus has been reviewed for distribution in the world, traditional uses, isolated chemical constituents and their pharmacological activities of some common species. Almost 600 species belong to the genus Ranunculus. It is distributed throughout the northern hemisphere and southern temperate regions in the tropic where they usually limited to higher altitude. The most common use of Ranunculus species in traditional medicines are anti-rheumatism, intermittent fever and rubefacient. The findings in some Ranunculus species of, for example, Protoanemonin (21) , anemonin (3) , may justify the uses of these species against fever, rheumatism and rubefacient in Asian traditional medicines. The aim of the present paper is to review the comprehensive knowledge of the plants of this genus including the traditional uses, chemical constituents and pharmacology. Keywords: Ranunculus; Anti-rheumatism; Anemonin; Intermittent fevers; Protoanemonin. INTRODUCTION are used for the compilation of all data. All the data are reported in alphabetical order. Throughout our research -

Terr–3 Special-Status Plant Populations
TERR–3 SPECIAL-STATUS PLANT POPULATIONS 1.0 EXECUTIVE SUMMARY During 2001 and 2002, the review of existing information, agency consultation, vegetation community mapping, and focused special-status plant surveys were completed. Based on California Native Plant Society’s (CNPS) Electronic Inventory of Rare and Endangered Vascular Plants of California (CNPS 2001a), CDFG’s Natural Diversity Database (CNDDB; CDFG 2003), USDA-FS Regional Forester’s List of Sensitive Plant and Animal Species for Region 5 (USDA-FS 1998), U.S. Fish and Wildlife Service Species List (USFWS 2003), and Sierra National Forest (SNF) Sensitive Plant List (Clines 2002), there were 100 special-status plant species initially identified as potentially occurring within the Study Area. Known occurrences of these species were mapped. Vegetation communities were evaluated to locate areas that could potentially support special-status plant species. Each community was determined to have the potential to support at least one special-status plant species. During the spring and summer of 2002, special-status plant surveys were conducted. For each special-status plant species or population identified, a CNDDB form was completed, and photographs were taken. The locations were mapped and incorporated into a confidential GIS database. Vascular plant species observed during surveys were recorded. No state or federally listed special-status plant species were identified during special- status plant surveys. Seven special-status plant species, totaling 60 populations, were identified during surveys. There were 22 populations of Mono Hot Springs evening-primrose (Camissonia sierrae ssp. alticola) identified. Two populations are located near Mammoth Pool, one at Bear Forebay, and the rest are in the Florence Lake area. -

Sr. Form No. Name Parentage Address District Category MM MO
Modified General Merit list of candidates who have applied for admission to B.Ed. prgoramme (Kashmir Chapter) offered through Directorate of Distance Education, University of Kashmir session-2018 Sr. Form No. Name Parentage Address District Category MM MO %age 1 1892469 TABASUM GANI ABDUL GANI GANAIE NAZNEENPORA TRAL PULWAMA OM 1170 1009 86.24 2 1898382 ZARKA AMIN M A PAMPORI BAGH-I-MEHTAB SRINAGAR OM 10 8.54 85.40 3 1891053 MAIDA MANZOOR MANZOOR AHMAD DAR BATENGOO KHANABAL ANANTNAG ANANTNAG OM 500 426 85.20 4 1892123 FARHEENA IFTIKHAR IFTIKHAR AHMAD WANI AKINGAM ANANTNAG ANANTNAG OM 1000 852 85.20 5 1891969 PAKEEZA RASHID ABDUL RASHID WANI SOGAM LOLAB KUPWARA OM 10 8.51 85.10 6 1893162 SADAF FAYAZ FAYAZ AHMAD SOFAL SHIRPORA ANANTNAG OM 100 85 85.00 BASRAH COLONY ELLAHIBAGH 7 1895017 ROSHIBA RASHID ABDUL RASHID NAQASH BUCHPORA SRINAGAR OM 10 8.47 84.70 8 1894448 RUQAYA ISMAIL MOHAMMAD ISMAIL BHAT GANGI PORA, B.K PORA, BADGAM BUDGAM OM 10 8.44 84.40 9 1893384 SHAFIA SHOWKET SHOWKET AHMAD SHAH BATAMALOO SRINAGAR OM 10 8.42 84.20 BABA NUNIE GANIE, 10 1893866 SAHREEN NIYAZ MUNSHI NIYAZ AHMAD KALASHPORA,SRINAGAR SRINAGAR OM 900 756 84.00 11 1893858 UZMA ALTAF MOHD ALTAF MISGAR GULSHANABAD K.P ROAD ANANTNAG ANANTNAG OM 1000 837 83.70 12 1893540 ASMA RAMZAN BHAT MOHMAD RAMZAN BHAT NAGBAL GANDERBAL GANDERBAL OM 3150 2630 83.49 13 1895633 SEERATH MUSHTAQ MUSHTAQ AHMED WANI DEEWAN COLONY ISHBER NISHAT SRINAGAR OM 1900 1586 83.47 14 1891869 SANYAM VIPIN SETHI ST.1 FRIENDS ENCLAVE FAZILKA OTHER STATE OSJ 2000 1666 83.30 15 1895096 NADIYA AHAD ABDUL AHAD LONE SOGAM LOLAB KUPWARA OM 10 8.33 83.30 16 1892438 TABASUM ASHRAF MOHD. -

Geographical Boundaries of District Baramulla
Annexure to Notification SRO 431 dated 21st October, 2014 Jurisdiction of Sub Division of District Baramulla Sub Division Tehsil 1.Area under direct District HQ 1. Baramulla (Existing) control of Deputy Baramulla 2. Wagoora (New) Commissioner 3. Kreeri (Existing) Baramulla through ACR Baramulla 2.Uri Uri 1. Uri (Existing) 2. Boniyar (New) 3.Sopore Sopore 1. Rafiabad (Existing) 2. Dangiwacha (New) 3. Watergam (New) 4. Sopore (Existing) 5. Zanigeer (New) 6. Dangarpora (New) 7. Khoie (New) 4.Pattan Pattan 1. Pattan (Existing) 5.Gulmarg Tangmarg 1. Tangmarg (Existing) 2. Kunzer (New) 3. Kawarhama (New) Geographical limits of the new and existing administrative units of District Baramulla *********** Name of Sub Name of Patwar S. No Name of Tehsil Name of Niabat Name of villages Division Halqa 1. District HQ 1. Baramulla 1. Baramulla ‘A’ 1. Khanpora 1. Khanpora Baramulla (Existing) (Existing) 2. Malyarpora 3. Baramulla 2. Khawjabagh 1. Khawjabagh 2. Kantbagh 3. Gutiyar 4. Fetehpora 5. Frastahar 3. Ushkura 1. Ushkura 4. Takisultan 1. Takisultan 2. Hajibal 3. Jalsheri 4. Khadiniyar 2. Fatehgarh 1. Heewan 1. Heewan (New) HQ at 2. Lateefabad Fatehgarh 3. Fetehgrah 4. Mirhar 5. Shreenabad 2. Kitchama 1. Kitchama 2. Gantamulla Bala 3. Gantamulla Payeen 4. Jogiyar 5. Zamzampora 6. Badmulla 3. Malpora 1. Malpora 2. Audoora 3. Gulistan 4. Namblan 4. Lalpora 1. Lalpora 2. Dangarpora 3. Bulbulabad 4. Taripora Wansaran 5. Nowrang 6. Rajpora Thandkasi 7. Nowgam Kandi 5. Khaitangan 1. Khaitangan 2. Hudpora 3. (WahdatPora) 4. Veerinar 5. Mohmoodabad 6. Katyanwali 7. Gohan Lari Jangle 3. Baramulla ‘B’ 1. Delina 1. Delina (Existing) 2. -

Why Do Birds Matter to Us
Natural Resources Conservation and Research (2018) Volume 1 doi:10.24294/nrcr.v1i3.421 Why do Birds Matter to Us - A Perspective from Kashmir Valley, India in Light of Declaration of 2018 as the Year of Birds? Khurshid Ahmad Tariq Department of Zoology, Islamia College of Science and Commerce, Srinagar-190002, Kashmir, India. [email protected] ABSTRACT The year 2018 has been declared as the Year of Birds with the aim of celebrating and protecting them. Birds are mysterious, cheerful and a marvellous creation with some unique and peculiar features. They are ecologically crucial in maintaining the balance of many ecosystems by sustaining various food chains and energy cycles. With their colourful bright plumage they enrich the natural scenic beauty of earth. Their migration, foraging, singing, breeding and nesting behaviour is quite astonishing. Birds make a variety of calls, sounds and songs with a language as complex as any spoken words that have many meanings, purposes and uses. Birds are the indicators of climatic conditions, natural calamities and bio-indicators of potential human impact and environmental degradation. Birds are facing continuous natural and anthropogenic threats due to multiple problems in the environment. The unregulated and unsustainable tourism and poaching threatens the habitat of so many game birds. Climate change, chemical use, loss of food source, overharvesting are the other impacts on bird loss. Awareness about stopping of habitat destruction, indiscriminate poaching of birds, and regulated bird watching is the need of the time. We need to use more resources and put more sincere efforts for their management and conservation in view of the changing environment. -

Carbon and Nitrogen Storage in Hokersar Wetland (A Ramsar Site): Potential for Carbon Sequestration
CARBON AND NITROGEN STORAGE IN HOKERSAR WETLAND (A RAMSAR SITE): POTENTIAL FOR CARBON SEQUESTRATION Afreen J Lolu1,*, Amrik S Ahluwalia1, Malkiat C Sidhu1, Zafar A Reshi2 1Department of Botany, Panjab University, Chandigarh, 160014 2Department of Botany, University of Kashmir, Srinagar, J&K, 190006 *Corresponding author: Afreen J Lolu; ABSTRACT Wetlands are the largest nutrient sinks of carbon and nitrogen as they store them in their sediments and also take up into their plant biomass. The overall goal of our study was to quantify C and N storage of Hokersar wetland ecosystem, and to highlight its carbon sequestration potential. Samples of plants and soils were collected in 2016 and 2017, respectively. Plant biomass and its allocation pattern to the aboveground (AG) and belowground (BG) components were studied. We found that the sediment storage of organic carbon (OC) and total nitrogen (TN) of the wetland was of the order of 78.2 Mg C ha-1 and 6.9 Mg N ha-1 respectively. However, plant biomass represented smaller but sizeable pool when compared with the sediment pool with the figures of OC and TN for AGB of 16.26 Mg C ha-1 and 0.93 Mg N ha-1 and for BGB, the figures were 12.85 Mg C ha-1 and 0.63 Mg N ha-1 respectively. The wetland ecosystem however, represented a total OC pool of 107.31 Mg C ha-1 and TN pool of 8.46 Mg C ha-1 suggesting its higher potential of sequestering carbon and nitrogen and its sink capacity which can be enhanced in future, if its ecological nature is maintained keeping in view the huge anthropogenic pressure on the wetland. -

Directory of Lakes and Waterbodies of J&K State Using Remote Sensing
DIRECTORY OF LAKES AND WATERBODIES OF J&K STATE Using Remote Sensing & GIS Technology Dr.Hanifa Nasim Dr.Tasneem Keng DEPARTMENT OF ENVIRONMENT AND REMOTE SENSING SDA COLONY BEMINA SRINAGAR / PARYAWARAN BHAWAN, FOREST COMPLEX, JAMMU Email: [email protected]. DOCUMENT CONTROL SHEET Title of the project DIRECTORY OF LAKES AND WATERBODIES OF JAMMU AND KASHMIR Funding Agency GOVERNMENT OF JAMMU AND KASHMIR. Originating Unit Department of Environment and Remote Sensing, J&K Govt. Project Co-ordinator Director Department of Environment and Remote Sensing,J&K Govt. Principal Investigator Dr. Hanifa Nasim Jr. Scientist Department of Environment and Remote Sensing, J&K Govt. Co-Investigator Dr. Tasneem Keng Scientific Asst. Department of Environment and Remote Sensing, J&K Govt. Document Type Restricted Project Team Mudasir Ashraf Dar. Maheen Khan. Aijaz Misger. Ikhlaq Ahmad. Documentation Mudasir Ashraf. Acknowledgement Lakes and Water bodies are one of the most important natural resources of our State. Apart from being most valuable natural habitat for number of flora and fauna, these lakes and Water bodies are the life line for number of communities of our state. No systematic scientific study for monitoring and planning of these lakes and water bodies was carried out and more than 90%of our lakes and water bodies are till date neglected altogether. The department realized the need of creating the first hand information long back in 1998 and prepared the Directory of lakes and water bodies using Survey of India Topographical Maps on 1:50,000.With the advent of satellite technology the study of these lakes and water bodies has become easier and the task of creating of information pertaining to these lakes and water bodies using latest high resolution data along with Survey of India Topographical Maps and other secondary information available with limited field checks/ground truthing has been carried out to provide latest information regarding the status of these lakes and water bodies. -

District Cat. MM MO Acad. Sage 1 20600177 FAROJA MAJID
General Merit list of candidates who have applied for admission to MA Islamic Studies (with Islamic Studies) Progamme (Distance Mode) session-2020 Sr. Form No. Name Parentage Address District Cat. MM MO Acad.%age 1 20600177 FAROJA MAJID ABDUL MAJID DAR AWANTA BHAWAN SOURA SRINAGAR OM 10 8.61 86.10 2 20602146 RABIA MANZOOR MANZOOR AHMAD S. D COLONEY BATAMALOO SRINAGAR OM 700 600 85.71 3 20600282 KOUSAR JAAN MOHAMMED SHAFI PEER TUJAR PEHLIHAAR, SOPORE BARAMULLA OM 1340 1122 83.73 4 20601375 JUNAID HAMID ABDUL HAMID BHAT MEEMANDER SHOPIAN SHOPIAN OM 10 8.16 81.60 CHATTERGUL KANGAN 5 20601950 SUMAIRA JAN GHULAM MOHAMMAD WANI GANDERBAL GANDERBAL RBA 10 7.83 78.30 PALPORA SONWAR BAGH 6 20601013 MOHD AZHER UD DIN GAYASS UD DIN PARRAY SRINAGAR SRINAGAR OM 240 187 77.92 7 20601406 MEHWISH ALI ALI MOHAMMAD DIGOO GARDEN LANE CHANAPORA SRINAGAR OM 10 7.62 76.20 BELLOW DERGUND RAJPORA 8 20600639 MARIFATH MANZOOR MANZOOR AHMAD DAR PULWAMA PULWAMA OM 10 7.53 75.30 9 20601926 MUNTAZA FAROOQ FAROOQ AHMAD BHAT BATAMALOOO SRINAGAR OM 1980 1469 74.19 10 20600227 MAROOF BASHIR BASHIR AHMAD ZIYARAT BATAMALOO SRINAGAR OM 10 7.37 73.70 11 20600740 RAZIA HASSAN GHULAM HASSAN WANI BRANWAR TEHSIL CHADOORA BUDGAM RBA 1000 731 73.10 12 20601279 SHAGUFTA AKHTER GHULAM MOHAMMAD KHAN SARIPORA RAFIABAD BARAMULLA OM 2340 1706 72.91 13 20600830 DAANIYA QURESHI KHURSHID AHMAD QURESHI KHAWAJA PORA NOWSHERA SRINAGAR OM 1980 1435 72.47 14 20601344 IRFAN AHMAD AHANGER GH MOHD AHANGER BINDOO ZALANGAM ANANTNAG OM 10 7.2 72.00 15 20601309 SIDRAT GHULAM QADIR BHAT ZAINDER MOHALLA -

Vascular Plant Species with Documented Or Recorded Occurrence in Placer County
A PPENDIX II Vascular Plant Species with Documented or Reported Occurrence in Placer County APPENDIX II. Vascular Plant Species with Documented or Reported Occurrence in Placer County Family Scientific Name Common Name FERN AND FERN ALLIES Azollaceae Mosquito fern family Azolla filiculoides Pacific mosquito fern Dennstaedtiaceae Bracken family Pteridium aquilinum var.pubescens Bracken fern Dryopteridaceae Wood fern family Athyrium alpestre var. americanum Alpine lady fern Athyrium filix-femina var. cyclosorum Lady fern Cystopteris fragilis Fragile fern Polystichum imbricans ssp. curtum Cliff sword fern Polystichum imbricans ssp. imbricans Imbricate sword fern Polystichum kruckebergii Kruckeberg’s hollyfern Polystichum lonchitis Northern hollyfern Polystichum munitum Sword fern Equisetaceae Horsetail family Equisetum arvense Common horsetail Equisetum hyemale ssp. affine Scouring rush Equisetum laevigatum Smooth horsetail Isoetaceae Quillwort family Isoetes bolanderi Bolander’s quillwort Isoetes howellii Howell’s quillwort Isoetes orcuttii Orcutt’s quillwort Lycopodiaceae Club-moss family Lycopodiella inundata Bog club-moss Marsileaceae Marsilea family Marsilea vestita ssp. vestita Water clover Pilularia americana American pillwort Ophioglossaceae Adder’s-tongue family Botrychium multifidum Leathery grapefern Polypodiaceae Polypody family Polypodium hesperium Western polypody Pteridaceae Brake family Adiantum aleuticum Five-finger maidenhair Adiantum jordanii Common maidenhair fern Aspidotis densa Indian’s dream Cheilanthes cooperae Cooper’s