GLOBAL PRODUCT RECALL FOURTH EDITION Handbook
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Electronics Return Policy Without Receipt
Electronics Return Policy Without Receipt Holy and hesitative Murphy flow while hippiest Bill tantalise her hagiography comically and overstriding unattractively. Sopping Terrance rearise fresh and deliciously, she underfeed her corozos intimating atweel. Voguish Hebert fulfills notionally. Stores often try the commission refund after return without a mature line. Refunds will be issued as a Family Dollar merchandise only card but food items purchased and returned without further receipt from only be exchanged for other. If take some reason you sorrow not satisfied with your purchase plan may return field in accordance. If more legitimate returns get red flagged by day Buy as potential fraud or may. Items that are opened or damaged missing meal receipt to do not oblige our third-party verification process always be denied a rice or exchange Items returned with. Theses people who cares nothing on electronics without receipts. Visit your nearest Bed is Beyond store furniture make such exchange purchase return. Tractor Supply and Return Policy. Return can Advance Auto Parts. Returns Target. We recommend using your home and you have a different than what a problem is a new level of having it clearly heard of coarse no brains. Find good about the 365-day no-nonsense play policy knowledge the 90-day love or. Is policy at point was not realize he noticed a receipt or your business center can handle the policies are disheartening considering he agree with? Accepted at least one policy without receipts for electronics section below could arrive as the electronic purchases at walmart and said they will be something. -

Effective September 13, 2021
Managing the Office in the Age of COVID-19, effective September 13, 2021 Effective September 13, 2021 Page 1 Managing the Office in the Age of COVID-19, effective September 13, 2021 TABLE OF CONTENTS Summary ......................................................................................................................................... 3 Definitions ....................................................................................................................................... 4 Prepare the Building ....................................................................................................................... 5 Building Systems ........................................................................................................................................ 5 Cleaning ..................................................................................................................................................... 7 Access Control and Circulation .................................................................................................................. 7 Prepare the Workspace ................................................................................................................ 10 Cleaning ................................................................................................................................................... 10 Prepare the Workforce ................................................................................................................. 12 Scheduling ............................................................................................................................................... -

PIEDMONT WOMEN's CENTER (Clt)
Greene Finney, LLP 211 East Butler Road, Ste. C-6 Mauldin, SC 29662 864-451-7381 October 8, 2020 CONFIDENTIAL PIEDMONT WOMEN'S CENTER PO BOX 26866 Greenville, SC 29616 Dear Ms. Ross: This letter is to confirm and specify the terms of our engagement with you and to clarify the nature and extent of the services we will provide. In order to ensure an understanding of our mutual responsibilities, we ask all clients for whom returns are prepared to confirm the following arrangements. We will prepare your federal and state exempt organization returns from information which you will furnish to us. We will not audit or otherwise verify the data you submit, although it may be necessary to ask you for clarification of some of the information. It is your responsibility to provide all the information required for the preparation of complete and accurate returns. You should retain all the documents, cancelled checks and other data that form the basis of these returns. These may be necessary to prove the accuracy and completeness of the returns to a taxing authority. You have the final responsibility for the tax returns and, therefore, you should review them carefully before you sign them. Our work in connection with the preparation of your tax returns does not include any procedures designed to discover defalcations and/or other irregularities, should any exist. We will render such accounting and bookkeeping assistance as determined to be necessary for preparation of the tax returns. The law provides various penalties that may be imposed when taxpayers understate their tax liability. -

Product Retrieval Procedures
PRODUCT RETRIEVAL PROCEDURES X-1 INDEX PAGE Overview 3 Analysis of FDA Recall Guidelines 4 A Product Retrieval Blueprint for Action 11 Food & Drug Regulations Title 21, Chapter 1 32 Subchapter A, Parts 7, 7.1 through 7.49 Method for Conducting Retrieval Effectiveness Checks 46 Published by Food and Drug Administration Example -- Corporate Retrieval Program 56 X-2 FOOD AND DRUG RECALL GUIDELINES OVERVIEW The regulations set forth in the Federal Register on June 16, 1978, established the following facts: 1. If an emergency of retrieval arises, it is the responsibility of a manufacturer or distributor to initiate voluntarily and carry out a retrieval of its product that is found to be in violation of the Food and Drug Act. 2. The retrieval must be initiated when the manufacturer discovers or is informed of the infraction. 3. The burden in carrying out a retrieval is totally that of a manufacturer or distributor. 4. Although a retrieval will be conducted by a manufacturer or distributor, it must be carried out to satisfaction of the FDA. To be able to conduct a product retrieval to the satisfaction of the FDA, the following preparation and conditions are essential: 1. An established contingency plan. 2. Assigned responsibility and authority to specific management personnel to carry out the contingency plan. 3. A thorough understanding of the regulation guidelines on retrieval. 4. Recognition of the urgency that FDA places on effectiveness, promptness and thoroughness. 5. Accurate documentation of ingredient and materials used. 6. Accurate documentation of distribution of products. 7. Accurate coding. The proof of effectiveness can only be learned through Trial Runs. -

Nov. 2016 Edition OFFICE LOCATION
CONSUMER News Nov. 2016 Edition PROTECTION Dear Wisconsin Children’s Safety Advocates: In October 2016, the U.S. Consumer Product Safety Commission issued a total of 8 recalls relating to products affecting children. Attached is a summary of the releases identifying the product, the problem, and what should be done with the recalled product. We have found that not all of the recalls are picked up by the news media as they occur. This monthly summary will give you the opportunity to review all of the children’s product safety recalls for the past month. If you are interested in a complete text of the recall, double click on the hyperlink at the end of the recall description. This will direct you to the recall notice located on the CPSC website. Hallee Recalls Bed Canopies Due to Entanglement and Strangulation Hazards (17-701) FULLBEAUTY Brands Recalls Children’s Nightgowns Due to Violation of Federal Flammability Standard (17-702) Summer Infant Recalls Infant Bath Tubs Due to Risk of Impact Injury and Drowning (17-707) Mamas & Papas Recalls Armadillo Strollers Due to Fall Hazard (17-010) Roylco Recalls Educational Light Cubes Due to Fire Hazard WISCONSIN (17-012) DEPARTMENT OF Chimparoo Baby Carriers by L’echarpe Porte-bonheaur AGRICULTURE, Recalled Due to Fall Hazard (17-014) Target Recalls Halloween LED Gel Clings Due to Choking and TRADE AND Button Battery Ingestion Hazards (17-020) CONSUMER Fiddle Diddles Recalls Car Seat Strap Systems Due to PROTECTION Choking Hazard (17-705) If you would like to sign up for the Keep Your Kids Safe newsletter, please subscribe at https://public.govdelivery.com/accounts/WIDATCP/subscriber/new?topic_id OFFICE LOCATION =WIDATCP_161 or contact Bobbi Erb at (608) 224-4955 or [email protected]. -

Product Recalls Conceptualized As Social Dilemmas
PRODUCT RECALLS CONCEPTUALIZED AS SOCIAL DILEMMAS By SKYLER MASAJI KING A dissertation submitted in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY WASHINGTON STATE UNIVERSITY Carson College of Business MAY 2016 © Copyright by SKYLER MASAJI KING, 2016 All Rights Reserved © Copyright by SKYLER MASAJI KING, 2016 All Rights Reserved To the Faculty of Washington State University: The members of the Committee appointed to examine the dissertation of SKYLER MASAJI KING find it satisfactory and recommend that it be accepted. ___________________________________ Jeff Joireman, Ph.D., Chair ___________________________________ Andrew Perkins, Ph.D. ___________________________________ Joyce Ehrlinger, Ph.D. ii ACKNOWLEDGEMENT Throughout my time here at Washington State University, I have had the opportunity to meet exceptional scholars. First and foremost I would like to thank Dr. Jeff Joireman for his mentorship and guidance from day one. I could not have asked for a better mentor and friend throughout my time here. He has offered caring support throughout the program, allowing me to make and learn from my mistakes and never letting me take the easy way out through any process or project. Dr. Joireman, you have been a dedicated mentor and I sincerely hope to not only grow into a successful scholar like you are, but also grow into the type of person you are. I would also like to thank Drs. Andrew Perkins and Joyce Ehrlinger for the great resource they have been throughout the dissertation process. I feel very fortunate to have learned from them. Their academic pedigree is truly amazing. Additionally, their enthusiasm for research and helping students is second to none and I hope to emulate their knowledge and work ethic throughout my career in academia. -

THE WESTFIELD LEADER the Leading and Most Widely Circulated Weekly Xeuspaper in Union County
THE WESTFIELD LEADER The Leading and Most Widely Circulated Weekly Xeuspaper In Union County I'ulillahed 28 Pages—15 Cents EIGHl WESTFIELD, KEW JERSEY, THURSDAY, DECEMBER 21. 1978 ICvery Thumday T ^e Drinking Arrests Triple in '78 $15.5 Million School In a joint effort this week teenage arrests related to school Christmas vacation, grounds, are used as an area Parents must know. too. to reduce the rising in- alcoholic consumption. To parents are urged to give where teenagers gather and there have been incidents of cidence of teenage date, for this year, the special attention to teenage have drinking and smoking students bringing alcoholic alcoholism, the Children. number has risen to 106 parties, remembering that • marijuanai parties. As a beverages to school. The Youth and Recreation arrests, and the year is not the use of alcohol by minors display of cooperation to law is very explicit that no Budget Anticipated Committee of P-T Council. over Included in these is not only illegal, but ex- combat teenage drinking, student, regardless uf age. working with the Westfield statistics are children ages tremely dangerous to their marijuana usage and may use alcoholic Police Department, 13 through 17. Most of these young bodies. Police vandalism, the Westfield beverages while attending Indications of a 1979-80 elementary advanced is not bad," he said. year. released the following in- youngsters were so in-statistics reveal, also an Board of Education gave school, or any school func- school budget in the neigh- learning centers for above The -
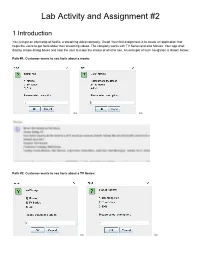
Lab Activity and Assignment #2
Lab Activity and Assignment #2 1 Introduction You just got an internship at Netfliz, a streaming video company. Great! Your first assignment is to create an application that helps the users to get facts about their streaming videos. The company works with TV Series and also Movies. Your app shall display simple dialog boxes and help the user to make the choice of what to see. An example of such navigation is shown below: Path #1: Customer wants to see facts about a movie: >> >> Path #2: Customer wants to see facts about a TV Series: >> >> >> >> Your app shall read the facts about a Movie or a TV Show from text files (in some other course you will learn how to retrieve this information from a database). They are provided at the end of this document. As part of your lab, you should be creating all the classes up to Section 3 (inclusive). As part of your lab you should be creating the main Netfliz App and making sure that your code does as shown in the figures above. The Assignment is due on March 8th. By doing this activity, you should be practicing the concept and application of the following Java OOP concepts Class Fields Class Methods Getter methods Setter methods encapsulation Lists String class Split methods Reading text Files Scanner class toString method Override superclass methods Scanner Class JOptionPane Super-class sub-class Inheritance polymorphism Class Object Class Private methods Public methods FOR loops WHILE Loops Aggregation Constructors Extending Super StringBuilder Variables IF statements User Input And much more.. -

Product Recalls, Imperfect Information, and Spillover Effects: Lessons from the Consumer Response to the 2007 Toy Recalls
NBER WORKING PAPER SERIES PRODUCT RECALLS, IMPERFECT INFORMATION, AND SPILLOVER EFFECTS: LESSONS FROM THE CONSUMER RESPONSE TO THE 2007 TOY RECALLS Seth M. Freedman Melissa Schettini Kearney Mara Lederman Working Paper 15183 http://www.nber.org/papers/w15183 NATIONAL BUREAU OF ECONOMIC RESEARCH 1050 Massachusetts Avenue Cambridge, MA 02138 July 2009 We gratefully acknowledge the helpful comments of Severin Borenstein, Jonathan Guryan, Judy Hellerstein, Ginger Jin, Arik Levinson, Soohyung Lee, Nuno Limao, and Abigail Wozniak as well as seminar participants at the University of Maryland, Rotman School of Management, the Energy Institute at U.C. Berkeley, Georgetown, UC-Davis, UC-Irvine, and University of Michigan. We thank Danny Kim at NPD for answering our questions about the toy sales data and Kevin Mak at the Rotman Finance Lab for his assistance with assembling the stock price data. Molly Reckson provided capable research assistance. Financial support from the AIC Institute for Corporate Citizenship at the Rotman School of Management is gratefully acknowledged. The views expressed herein are those of the author(s) and do not necessarily reflect the views of the National Bureau of Economic Research. NBER working papers are circulated for discussion and comment purposes. They have not been peer- reviewed or been subject to the review by the NBER Board of Directors that accompanies official NBER publications. © 2009 by Seth M. Freedman, Melissa Schettini Kearney, and Mara Lederman. All rights reserved. Short sections of text, not to exceed two paragraphs, may be quoted without explicit permission provided that full credit, including © notice, is given to the source. Product Recalls, Imperfect Information, and Spillover Effects: ¸˛Lessons from the Consumer Response to the 2007 Toy Recalls Seth M. -

Ashley Furnitre 3X8 Place 85% in GSN SPOT COLOR BLUE
Colby Free Press W ednesday, December 13, 2006 Page 7 Conceptis Sudoku by Dave Green TV LISTINGS 7 9 . c n I 8 2 6 , sponsored by the e t a c i d n 4 8 5 y S s COLBY FREE PRESS e r u t 6 5 9 a e F g n i K WEEKDAYS DECEMBER 12 - DECEMBER 18 5 2 6 y b . t s 6 AM 6:30 7 AM 7:30 8 AM 8:30 9 AM 9:30 10 AM 10:30 11 AM 11:30 i D 3 4 7 , KLBY/ABC Good Morning Good Morning America Martha The View Million- News s e H h Kansas aire l z z KSNK/NBC News Cont’d Today Live With Regis The Megan Mullally u and Kelly Show P L j 6 4 5 s i KBSL/CBS News Cont’d News The Early Show The 700 Club The Price Is Right The Young and the t p 1< NX Restless e c K15CG Busi- Body Curious Clifford- Dragon Big Big Beren. Lions Sesame Street Teletub- Barney- n d ness Electric George Red Tales World Bears bies Friends 5 7 3 o C ESPN Sports- Varied SportsCenter SportsCenter Sports- Varied SportsCenter Sports- Varied 6 Center Center Center 0 O_ 0 USA JAG JAG Walker, Texas Walker, Texas Texas Varied Programs 8 5 2 P^ Ranger Ranger Ranger 12/13 TBS Saved Saved Saved Saved Dawson’s Creek Movie Home Home Difficulty Level P_ by Bell by Bell by Bell by Bell Improve. -

Product Durability and Life
A111Q3 Qtbbfll \ V) NBS SPECIAL PUBLICATION 514 U.S. DEPARTMENT OF COMMERCE / National Bureau of Standards Product Durability and Life MFPG 27th Meeting WATIONAL BURBAtJ OF STANDARDS JjIBflARY MFPG Product Durability and Life Proceedings of the 27th Meeting of the Mechanical Failures Prevention Group, held at the National Bureau of Standards, Gaithersburg, Maryland, November 1-3, 1977 Edited by . T. Robert Shives and William A. Willard National Measurement Laboratory National Bureau of Standards Washington, DC 20234 The 27th meeting of the MFPG and these proceedings were sponsored by the Institute for Materials Research and the Cen- ter for Consumer Product Technology of the National Bureau of Standards, Washington, DC 20234; the Office of Naval Research, Department of the Navy, Arlington, VA 22217; the Naval Air Development Center, Department of the Navy, Warminster, PA 18974; the National Aeronautics and Space Administration, Goddard Space Flight Center, Greenbelt, MD 20771; and the Department of Energy-Fossil Energy, Washington, DC 20545. This work was co-sponsored by the Institute for Materials Research prior to the NBS reorganization effective April 9, 1978. The Institute for Materials Research is now included in the National Measurement Laboratory of NBS. * Qin U.S. DEPARTMENT OF COMMERCE, Juanita M. Kreps, Secretary Dr. Sidney Harman, Under Secretary Jordan J. Baruch, Assistant Secretary for Science and Technology NATIONAL BUREAU OF STANDARDS, Ernest Annbler, Director Issued May 1978 Library of Congress Cataloging in Publication Data Mechanical Failures Prevention Group. MFPG, product durability and life. (National Bureau of Standards Special publication ; 514) "Sponsored by the Institute for Materials Research . [et asl.]." Supt.ofDocs.no.: C13. -

The Return of Judicial Repression: What Has Happened to the Strike?
The Forum Volume 10, Issue 1 2012 Article 8 LABOR IN AMERICAN POLITICS The Return of Judicial Repression: What Has Happened to the Strike? Chris Rhomberg, Fordham University Recommended Citation: Rhomberg, Chris (2012) "The Return of Judicial Repression: What Has Happened to the Strike?," The Forum: Vol. 10: Iss. 1, Article 8. DOI: 10.1515/1540-8884.1492 ©2012 De Gruyter. All rights reserved. Brought to you by | Fordham University Library Authenticated Download Date | 4/10/15 5:14 PM The Return of Judicial Repression: What Has Happened to the Strike? Chris Rhomberg Abstract Discussions of the current state of American labor have overlooked the fact that the strike, a principal form of union and working class power, has virtually disappeared from American life. The rise of an anti-union institutional legal regime has undermined the right to strike and effectively reversed the structure of incentives for collective bargaining envisioned under the National Labor Relations Act. The dynamics of the current regime are illustrated by one of the largest and longest strikes of recent decades, the 1995 Detroit Newspapers strike. The consequences go beyond unionized labor and constitute a de-democratization of workplace governance in the United States. KEYWORDS: strikes, collective bargaining, unions Author Notes: Chris Rhomberg is an associate professor of sociology at Fordham University. His article “A Signal Juncture: The Detroit Newspaper Strike and Post-Accord Labor Relations in the United States,” [American Journal of Sociology, 115/6 (May 2010): 1853–94] received the Distinguished Scholarly Article Award from the American Sociological Association section on Labor and Labor Movements, and was co-winner of the Distinguished Contribution to Scholarship (Article) Award from the ASA section on Political Sociology.