Distribution Pattern, Threatened Status and Conservation Measures Of
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Department Activities
DEPARTMENT ACTIVITIES 59 DEPARTMENT ACTIVITIES ECE Forum Mech Forum Civil Forum EEE Forum Readers Club FDP INAUGURATION OF CHEMISTRY LABORATORY T h e C h e m i s t r y L a b o r a t o r y w a s i n a u g u r a t e d b y Mr. M. Balasubramaniam, Managing Director, Sakthi Group of Companies and Governing Council Members of Sakthi Polytechnic College. At this juncture, our appreciation is due to the sincere efforts of Mr. P.R.Nagarajan, Lecturer (Sen. Gr.) and Mr. P.Dhanasekaran (Junior Drafting Officer) for their exclusive skill in designing and constructing the laboratories. Our appreciation is due to the staff members of the Department of Electrical and Electronics and Department of Mechanical Engineering. Further each and every department have submitted their proposal seeking grant from AICTE under MODROBS (Modernization & Removal of Obsolescence) DEPARTMENT ACTIVITIES FOR THE YEAR 2017 – 2018 DEPARTMENT OF CIVIL ENGINEERING Mr. K.R.Palanisamy , M.E., HOD Forum Meetings: Our department conducted various guest lecture during the year 2017 – 2018: 1. Er.D.Saptharishi B.E., Managing Director, Rishi Associates, Coimbatore delivered a guest lecture on the topic “Skillset for Civil Engineers” on 28.07.2017. 2. Er. M. Geo damin, M.E.,(Struct)Director (Design) &Rtn. Er. R. Kumaresh, B.E., (Civil Director (Techno - commercial) S.F. Engineering Systems (India) Pvt. Ltd Chennai delivered a guest lecture on the topic “ Pre-stressed concrete: An Overview” on 28.07.17 3. Dr.R.Muthuswamy M.E.,Ph.D.,MISTE.,MICI., Principal ,Sakthi Polytechnic college delivered a guest lecture on the topic “Earthquake Awareness” on 06.02.2018 Industrial Visits : Our department students visited various industries and organizations during the year 2017-2018: 1.Brick Chamber at T.N.Palayam & Bhavanisagar Dam on 11.07.17. -

Seasonal Variation of Cauvery River Due to Discharged Industrial Effluents at Pallipalayam in Namakkal
Vol. 8 | No. 3 |380 - 388 | July - September | 2015 ISSN: 0974-1496 | e-ISSN: 0976-0083 | CODEN: RJCABP http://www.rasayanjournal.com http://www.rasayanjournal.co.in SEASONAL VARIATION OF CAUVERY RIVER DUE TO DISCHARGED INDUSTRIAL EFFLUENTS AT PALLIPALAYAM IN NAMAKKAL K. Sneka Lata 1, A. Jesu 2, M.S. Dheenadayalan 1 1Department of Chemistry G.T.N. Arts College, Dindigul, Tamil Nadu. India. 2Department of Chemistry, Kathir College of Engineering, Neelambur, Coimbatore (T.N.)India *E-mail: [email protected] ABSTRACT The impact of industrial effluent like dyeing, sugar, and paper discharged from the banks of Cauvery river at pallipalayam in Namakkal district. It is observed during the study that many dyeing, sugar and paper units discharged their untreated effluent into the river Cauvery in this criminately without any treatment. The river water samples and ground water samples and soil sample collected in the study area reveals that high degree of the pollution cost by untreated effluent of heavy metal analysis from the river water and ground water and soil. So that industries major culprit in damaging the river water, ground water and soil used for the agricultural purpose. The increased loading of toxic effluent day by day due to the toxic effluent of surface water, ground water and soil. The total pollution due to industrial effluent causes the great damage to the environmental pollution of river Cauvery at pallipalayam in Namakkal district. Keywords: Raw effluents, treated effluents, total dissolved solids, dyeing industry, physico chemical analysis ©2015 RAS ĀYAN. All rights reserved INTRODUCTION The Kaveri, also spelled Cauvery in English, is a large Indian river. -

OCCASIO I AL PAPER O. 36 RECORDS of the ZOOLOGICAL SURVEY of INDIA
MISCELLANEOUS PUBLICATION OCCASIO I AL PAPER o. 36 RECORDS OF THE ZOOLOGICAL SURVEY OF INDIA MISCELLANEOUS PUBLICATION OCCASIONAL PAPER No. 36 A SURVEY OF THE CAUVERY RIVER SYSTEM WITH A MAJOR ACCOUNT OF ITS FISH FAUNA BY K. C. Jayaram Zoological Survey C!! India, Oalcutta-700 016 AND T~ Venkateswarlu" M. B. Ragunathan S.kern Regional Station, Zoological Survey of India, Madras 600 028 Edited by the Director, Zoological Survey. of India 1982 ® Copyright 1982, Government of India Published in August, 1982 PRICE: 1 nlana : Rs. 4~.OO Foreign : £ 6.00 $ 9,50 PRINTED ~N INDIA BY THB BANI PRESS, 16 HBMENDRA SBN STRBBT, CALCUTTA-700 006 AND PUBLISHED BY THB DIRBCTOR, ZOOLOGICAL SURVBY OP INDIA, CALCUTTA. RECORDS OF THE ZOOLOGICAL SURVEY OF INDIA Miscellaneous Publication Occasional Paper No. 36 1982 Pages 1-115 CONTENTS PAGE INTRODUCTION 1 WORK PROGRAMME ... 1 AUTHORSHIP ASSIGNMENTS 2 ACKNOWLEDGEMENTS 3 THE CAUVERY RIVER 3 CLIMATE AND VEGETATION 5 TRIBUTARIES 5 COLLECTING STATIONS WITH ECOLOGICAL NOTES 7 MARGINAL AND AQUATIC BIOTA 18 SYSTEMATIC LIST OF CAUVERY FISHES 20 SYSTEMATIC ACCOUNT ••• 28 DISCUSSION 107 CONCLUSIONS AND RECOMMENDATIONS 110 REFERENCES • • . , •• 112 INTRODUCTION Cauvery, Krishna and Godavary rivers constitute the major three ,1.er systems in South India. Geologically they are much older than die Oanga, Indus and Brahmaputra rivers of Northen India. The eco nomic prosperity of the southern states of Andhra Pradesh, Tamil Nadu Kerala and Karnataka is closely intertwined with the water-supply and potentialities of these three rivers. Since historical times their. waters have been extensively utilised for agriculture, fisheries, irrigation and tllYigation purposes. -

ERODE (Tamil Nadu) Issued On: 29-09-2021
India Meteorological Department Ministry of Earth Sciences Govt. of India Date: 29-09-2021 Block Level Forecast Weather Forecast of AMMAPET Block in ERODE (Tamil Nadu) Issued On: 29-09-2021 Wind Wind Cloud Date Rainfall Tmax Tmin RH Morning RH Evening Speed Direction Cover (Y-M-D) (mm) (°C) (°C) (%) (%) (kmph) (°) (Octa) 2021-09-30 0.5 32.6 21.5 81 39 4.0 281 8 2021-10-01 2.8 32.8 22.1 79 39 3.0 360 7 2021-10-02 2.4 31.6 22.1 86 46 6.0 72 7 2021-10-03 2.6 30.4 21.9 87 50 5.0 117 7 2021-10-04 1.7 30.4 21.8 87 51 4.0 122 5 Weather Forecast of ANDIYUR Block in ERODE (Tamil Nadu) Issued On: 29-09-2021 Wind Wind Cloud Date Rainfall Tmax Tmin RH Morning RH Evening Speed Direction Cover (Y-M-D) (mm) (°C) (°C) (%) (%) (kmph) (°) (Octa) 2021-09-30 2.9 31.1 20.9 81 39 4.0 293 8 2021-10-01 3.9 31.8 21.5 78 42 3.0 150 7 2021-10-02 16.2 31.0 21.5 89 47 5.0 68 7 2021-10-03 8.7 28.4 21.1 90 55 5.0 108 7 2021-10-04 16.6 28.9 20.9 91 55 5.0 114 6 Weather Forecast of BHAVANI Block in ERODE (Tamil Nadu) Issued On: 29-09-2021 Wind Wind Cloud Date Rainfall Tmax Tmin RH Morning RH Evening Speed Direction Cover (Y-M-D) (mm) (°C) (°C) (%) (%) (kmph) (°) (Octa) 2021-09-30 4.2 31.6 21.1 80 37 7.0 245 8 2021-10-01 2.2 32.0 21.8 75 38 4.0 180 7 2021-10-02 9.3 31.1 21.7 86 43 6.0 68 7 2021-10-03 3.8 29.2 21.4 86 50 6.0 107 7 2021-10-04 11.4 29.5 21.1 88 50 7.0 113 5 India Meteorological Department Ministry of Earth Sciences Govt. -

Contextual Water Targets Pilot Study Noyyal-Bhavani River Basin, India
CONTEXTUAL WATER TARGETS PILOT STUDY NOYYAL-BHAVANI RIVER BASIN, INDIA May 2019 Ashoka Trust for Research in Ecology and the Environment (ATREE) 1 Bangalore, India This publication is based on the project report submitted to the Pacific Institute, USA as the result of the study on contextual water targets in the Noyyal-Bhavani river basin, India. Study duration: October 2018 to April 2019 Financial support: Pacific Institute, USA Additional financial support: World Wide Fund for Nature-India (WWF-India). Authors: Apoorva R., Rashmi Kulranjan, Choppakatla Lakshmi Pranuti, Vivek M., Veena Srinivasan Suggested Citation: R. Apoorva, Kulranjan, R., Pranuti, C. L., Vivek, M., and Srinivasan, V. 2019. Contextual Water Targets Pilot Study: Noyyal-Bhavani River Basin. Bengaluru. Ashoka Trust for Research in Ecology and the Environment (ATREE). Front-cover Photo Caption: Noyyal outflows from the Orathupalayam dam, which had become a reservoir of polluted water for years. Front-cover Photo Credit: Apoorva R. (2019) Back-cover Photo Caption: Untreated sewage in a drain flows towards the River Noyyal near Tiruppur city, Tamil Nadu Back-cover Photo Credit: Rashmi Kulranjan (2019) Acknowledgement: We are grateful to Mr. Ganesh Shinde from ATREE for his help and guidance related to land use classification and GIS maps in this project. We would like to thank all the participants of the project consultative meeting held in Coimbatore in March 2019 for sharing their ideas and contributing to the discussion. We are thankful to Ms. Upasana Sarraju for proofreading -

Download Download
OPEN ACCESS The Journaf of Threatened Taxa fs dedfcated to buffdfng evfdence for conservafon gfobaffy by pubffshfng peer-revfewed arfcfes onffne every month at a reasonabfy rapfd rate at www.threatenedtaxa.org . Aff arfcfes pubffshed fn JoTT are regfstered under Creafve Commons Atrfbufon 4.0 Internafonaf Lfcense unfess otherwfse menfoned. JoTT affows unrestrfcted use of arfcfes fn an y medfum, reproducfon, and dfstrfbufon by provfdfng adequate credft to the authors and the source of pubffcafon. Journaf of Threatened Taxa Buffdfng evfdence for conservafon gfobaffy www.threatenedtaxa.org ISSN 0974-7907 (Onffne) | ISSN 0974-7893 (Prfnt) Communfcatfon Freshwater ffsh fauna of Hfranyakeshf Rfver, the northern Western Ghats, Indfa Pradeep Kumkar, Sanjay S. Kharat, Nffn S. Sawant, Unmesh Katwate & Neefesh Dahanukar 26 May 2017 | Vof. 9| No. 5 | Pp. 10178–10186 10.11609/jott. 3126 .9.5 .10178-10186 For Focus, Scope, Afms, Poffcfes and Gufdeffnes vfsft htp://threatenedtaxa.org/About_JoTT For Arfcfe Submfssfon Gufdeffnes vfsft htp://threatenedtaxa.org/Submfssfon_Gufdeffnes For Poffcfes agafnst Scfenffc Mfsconduct vfsft htp://threatenedtaxa.org/JoTT_Poffcy_agafnst_Scfenffc_Mfsconduct For reprfnts contact <[email protected]> Pubffsher/Host Partner Threatened Taxa Journaf of Threatened Taxa | www.threatenedtaxa.org | 26 May 2017 | 9(5): 10178–10186 Freshwater ffsh fauna of Hfranyakeshf Rfver, Communfcatfon the northern Western Ghats, Indfa ISSN 0974-7907 (Onffne) Pradeep Kumka r 1 , Sanjay S. Kharat 2 , Nffn S. Sawant 3 , U nmesh Katwate 4 & ISSN 0974-7893 (Prfnt) Neefesh Dahanukar 5 OPEN ACCESS 1,2,3 Department of Zoofogy, Modern Coffege of Arts, Scfence and Commerce, Ganeshkhfnd, Pune, Maharashtra 411007, Indfa 4 Schoof of Ocean Scfence and Technofogy, Kerafa Unfversfty of Ffsherfes and Ocean Studfes (KUFOS), Kochf, Kerafa 682506, Indfa 4 Bombay Naturaf Hfstory Socfety (BNHS), Hornbfff House, Opp. -

Irrigation Projects of Tamil Nadu from 2001-2021
IRRIGATION PROJECTS OF TAMIL NADU FROM 2001-2021 NAME – VRINDA GUPTA INSTITUTION – K.R. MANGALAM UNIVERSITY 1 ABSTRACT From the ancient times water is always most important for agriculture purpose for growing crops. Since thousand years, humans have relied on agriculture to feed their communities and they have needed irrigation to water their crops. Irrigation includes artificially applying water to the land to enhance the growing of crops. Over the years, irrigation has come in many different forms in countries all over the world. Irrigation projects involves hydraulic structures which collect, convey and deliver water to those areas on which crops are grown. Irrigation projects unit may starts from a small farm unit to those serving extensive areas of millions of hectares. Irrigation projects consist of two types first a small irrigation project and second a large irrigation project. Small irrigation project includes a low diversion or an inexpensive pumping plant along with small channels and some minor control structures. Large irrigation project includes a huge dam, a large storage reservoir, hundreds kilometers of canals, branches and distributaries, control structures and other works. In this paper we discussing about irrigation plan of Tamil Nadu from 2001-2021. INTRODUCTION Water is the important or elixir of life, a precious gift of nature to humans and millions of other species living on the earth. It is hard to find in most part of the world. 4% of India’s land area in Tamil Nadu and inhabited by 6% of India’s population but water resources in India is only 2.5%. In Tamil Nadu, water is a serious limiting factor for agriculture growth which leads to irrigation reduces risk in farming, increases crop productivity, provides higher employment opportunities to the rural areas and increases farmer income. -

Noyyal-Bhavani River Basin, South India
Setting Site Water Targets Informed by Catchment Context CASE STUDY: Noyyal-Bhavani River Basin, South India July 2020 Project Team Sonali Abraham, Tien Shiao, and Abigail Warner UN Global Compact CEO Water Mandate www.ceowatermandate.org Pacific Institute www.pacinst.org Recommended Citation Abraham, Sonali, Tien Shiao, and Abigail Warner. 2020. Setting Site Water Targets Informed by Catchment Context, CASE STUDY: Noyyal-Bhavani River Basin, South India. United Nations Global Compact CEO Water Mandate and Pacific Institute. https://ceowatermandate.org/site-targets-guide/. Cover Photo: © Sergei Gussev/Flickr Support This project was generously supported by the CEO Water Mandate-endorsing companies that have engaged in the initiative’s India-focused work: Gap Inc., Levi Strauss & Co., and PVH Corp. Setting Site Water Targets Informed by Catchment Context Case Study: Noyyal-Bhavani River Basin, South India ISBN: 978-1-940148-03-8 Table of Contents Background: Setting Site Water Targets Informed by Catchment Context ..........4 Case Study: Noyyal-Bhavani River Basin ........................................6 The Pilot ..................................................................6 The Noyyal-Bhavani River Basin .............................................7 Elements for Setting Site Water Targets ......................................8 Element 1: Water targets should respond to priority water challenges within the catchment ...................................................8 Element 2: The ambition of water targets should be informed -
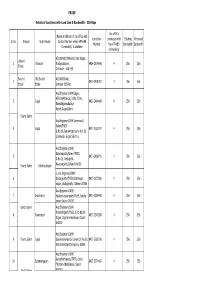
ERODE Sl.No Division Sub-Division Name & Address of the Office With
ERODE Details of Locations with Land Line & Bandwidth - 256 Kbps No. of PCs Name & Address of the office with Land Line connected with Existing Proposed Sl.No Division Sub-Division Contact Number where VPNoBB Number the VPNoBB Bandwidth Bandwidth Connectivity is available connectivity AE/O&M/S/Chithode,Indra Nagar, Urban / 1 Chithode Naduppalayam, 0424-2534848 4 256 256 Erode Chithode - 638 455 South / C&I/South/ AE/O&M/Solar, 2 0424-2401007 4 256 256 Erode Erode Iraniyan St,Solar Asst.Engineer,O&M/Gugai, AEE/O&M/Gugai, D.No.17/26 , 3 Gugai 0427-2464499 4 256 256 Ramalingamadalaya Street,Gugai,Salem Town/ Salem Asst.Engineer,O&M/ Linemedu/ Salem/TNEB 4 Gugai 0427-2218747 4 256 256 D.No.60,Ramalingamsamy Koil St, Linemedu Gugai Salem 6. Asst.Engineer,O&M/ Kalarampatty/Salem/TNEB, 5 0427-2468791 4 256 256 D.No.13, Nethaji St., Town/ Salem Kitchi palayam Kalarampatty,Salem 636015 Junior.Engineer,O&M/ 6 Dadagapatty/TNEB,Shanmuga 0427-2273586 4 256 256 nagar, dadagapatty Salem 636006 Asst.Engineer,O&M/ 7 Swarnapuri Mallamooppampatti/TNEB, Sundar 0427-2386400 4 256 256 nagar,Salem 636302 West/ Salem Asst.Engineer,O&M/ Narasothipatti/TNEB, 5/71-b2,PG 8 Swarnapuri 0427-2342288 4 256 256 Nagar, Jagirammapalayam.Salem 636302 Asst.Engineer,O&M/ 9 Town/ Salem Gugai Seelanaickenpatty/ Salem,SF.No.93, 0427-2281236 4 256 256 Seelanaickenpatty bypass, Salem Asst.Engineer,O&M/ 10 Suramangalam Rural/Nethimedu/TNEB, Circle 0427-2274466 4 256 256 Thottam /Nethimedu, Salem West/ Salem 636002 West/ Salem Asst.Engineer,O&M/ 11 Shevapet Kondalampatti/TNEB, 7/65 -

Erode District Disaster Management Plan - 2020
Erode District Disaster Management Plan - 2020 1 Erode District Disaster Management Plan - 2020 CHAPTER - 1 INTRODUCTION 1.1. Aims and Objectives of the District Disaster Management Plan: ➢ To engage in activities which may help in minimizing the damages caused by disasters in both urban and rural areas. ➢ To make endeavors towards creating awareness among the people about disasters and its consequences and to prepare them in advance to face such situations and to ensure their participation in the disaster mitigation plans. ➢ Existing institutional arrangements, interdepartmental linkages, role of NGOs, voluntary agencies and local communities so as to understand their capabilities to mitigate specific disasters which will also facilitate effective coordination in their activities in times of need. ➢ To act as an agency for the execution of disaster management schemes of the Government and the NGOs. ➢ To evolve information reporting and monitoring tools for preparedness, immediate response and damage assessment, keeping in view the socioeconomic conditions of urban and rural areas. 1.2. Authority for District Disaster Management Plan: In accordance with the ‘Section 30’ of the ‘Disaster Management Act, 2005’ Sub-Section (1) The District Authority shall act as the district planning; coordinating and implementing body for disaster management and take all measures for the purposes of disaster management in the district in accordance with the guidelines laid down by the National Authority and the State Authority. 1.3. Evolution of DDMP: Historically, emergency management and preparedness has been a reactive science. The District Magistrate who is the chief co-ordinator will be the focal point for coordinating all activities relating to prevention, mitigation and preparedness apart from his existing responsibilities pertaining to response and relief. -

– Kolab River 4)Indravati Dam – Indravati River 5)Podagada Dam – Podagada River 6)Muran Dam – Muran River 7)Kapur Dam – Kapur River
DAMS IN INDIA WEST BENGAL 1)FARRAKA BARRAGE – GANGES RIVER 2)DURGAPUR BARRAGE – DAMODAR RIVER 3)MAITHON DAM –BARAKAR RIVER 4)PANCHET DAM – DAMODAR RIVER 5)KANGSABATI DAM – KANGSABATI RIVER UTTAR PRADESH 1)RIHAND DAM – RIHAND RIVER 2)MATATILA DAM – BETWA RIVER 3)RAJGHAT DAM – BETWA RIVER ODISHA 1)HIRAKUND DAM – MAHANADI 2)RENGALI DAM – BRAHMANI RIVER 3)UPPER KOLAB DAMwww.OnlineStudyPoints.com – KOLAB RIVER 4)INDRAVATI DAM – INDRAVATI RIVER 5)PODAGADA DAM – PODAGADA RIVER 6)MURAN DAM – MURAN RIVER 7)KAPUR DAM – KAPUR RIVER www.OnlineStudyPoints.com DAMS IN INDIA JHARKHAND 1)MAITHON DAM- BARAKAR RIVER 2)PANCHET DAM- DAMODAR RIVER 3)TENUGHAT DAM – DAMODAR RIVER 5)GETALSUD DAM – SWARNAREKHA RIVER MADHYA PRADESH 1)GANDHISAGAR DAM – CHAMBAL RIVER 2)TAWA DAM – TAWA RIVER 3)INDIRA SAGAR DAM – NARMADA RIVER 4)OMKARESHWAR DAM – NARMADA RIVER 5)BARGI DAM – NARMADA RIVER 6)BARNA DAM – BARNA RIVER 7)BANSAGAR DAM – SON RIVER CHHATTISGARH www.OnlineStudyPoints.com 1)MINIMATA BANGO DAM – HASDEO RIVER 2)DUDHWA DAM – MAHANADI 3)GANGREL DAM – MAHANADI 4)SONDUR DAM – SONDUR 5)TANDULA DAM – TANDULA RIVER 6)MONGRA BARRAGE – SHIVNATH www.OnlineStudyPoints.com DAMS IN INDIA MAHARASHTRA 1)KOYNA DAM – KOYNA RIVER 2)JAYAKWADI DAM – GODAVARI RIVER 3)ISAPUR DAM – PENGANA RIVER 4)WARNA DAM – VARNA RIVER 5)TOTLADOH DAM – PENCH RIVER 6)SUKHANA DAM – SUKHANA RIVER 7)UJJANI DAM – BHIMA RIVER JAMMU AND KASHMIR 1)SALAL DAM – CHENAB RIVER 2)BAGLIHAR DAM – CHANAB RIVER 3)PAKUL DUL DAM – CHENAB RIVER 3)URI DAM – JHELUM RIVER 4)NIMBOO BAZGO HYDROELECTRIC PLANT – INDUS RIVER -

Habitat Preference and Biotic Pressure of Four Horned Antelope In
International Journal of Fauna and Biological Studies 2020; 7(4): 109-114 ISSN 2347-2677 www.faunajournal.com IJFBS 2020; 7(4): 109-114 Habitat preference and biotic pressure of four horned Received: 19-05-2020 antelope in Sathyamangalam Wildlife sanctuary, Tamil Accepted: 21-06-2020 Nadu, Southern India K Swamy Postgraduate and Research Department of Zoology and Wildlife Biology, A.V.C. College, K Swamy, M Karthikeyan and D Boominathan Mannampandal, Mayiladuthurai, Tamil Nadu, India Abstract Habitat preference of Four-horned Antelope Tetracerus quadricornis was studied at Sathyamangalam M Karthikeyan Postgraduate and Research Wildlife sanctuary. A grid based approach was followed to find out the habitat preference. Due to shy Department of Zoology and nature and small size of the animal make sightings difficult, so indirect signs also taken into Wildlife Biology, A.V.C. College, consideration when assessing their preference of habitat. Both direct sightings and indirect signs Mannampandal, Mayiladuthurai, locations were plotted and prepared a distribution map. The majority of the records (~72%) were on Tamil Nadu, India hill/nulla slopes; however this may be due to the terrain being largely composed of such areas. Flat and open areas had only 28% of the records. The encounter rate for signs/sightings was 1.71/km in the open D Boominathan habitat and it was much lower (0.25/km) in the fairly dense forests. Four-horned Antelopes largely prefer WWF-India Bhavanisagar, open habitat and appear to avoid dense areas. Tamil Nadu, India Keywords: Four horned antelope, Sathyamangalam, distribution, southern India Introduction Four horned Antelope (Tetracerus quadricornis) is only member of this group with two pairs of horns of which front pair is always shorter than the back (Prater, 1971) [9].