M7f Zeolite-Enclosed Micro-Cavities on Silicon Wafer
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Mg2sio4) in a H2O-H2 Gas DANIEL TABERSKY1, NORMAN LUECHINGER2, SAMUEL S
Goldschmidt2013 Conference Abstracts 2297 Compacted Nanoparticles for Evaporation behavior of forsterite Quantification in LA-ICPMS (Mg2SiO4) in a H2O-H2 gas DANIEL TABERSKY1, NORMAN LUECHINGER2, SAMUEL S. TACHIBANA1* AND A. TAKIGAWA2 2 2 1 , HALIM , MICHAEL ROSSIER AND DETLEF GÜNTHER * 1 Department of Natural History Sciences, Hokkaido 1ETH Zurich, Department of Chemistry and Applied University, N10 W8, Sapporo 060-0810, Japan. Biosciences, Laboratory of Inorganic Chemistry (*correspondence: [email protected]) (*correspondence: [email protected]) 2Carnegie Institution of Washington, Department of Terrestrial 2Nanograde, Staefa, Switzerland Magnetism, 5241 Broad Branch Road NW, Washington DC, 20015 USA. Gray et al. did first studies of LA-ICPMS in 1985 [1]. Ever since, extensive research has been performed to Forsterite (Mg2SiO4) is one of the most abundant overcome the problem of so-called “non-stoichiometric crystalline silicates in extraterrestrial materials and in sampling” and/or analysis, the origins of which are commonly circumstellar environments, and its evaporation behavior has referred to as elemental fractionation (EF). EF mainly consists been intensively studied in vacuum and in the presence of of laser-, transport- and ICP-induced effects, and often results low-pressure hydrogen gas [e.g., 1-4]. It has been known that in inaccurate analyses as pointed out in, e.g. references [2,3]. the evaporation rate of forsterite is controlled by a A major problem that has to be addressed is the lack of thermodynamic driving force (i.e., equilibrium vapor reference materials. Though the glass series of NIST SRM 61x pressure), and the evaporation rate increases linearly with 1/2 have been the most commonly reference material used in LA- pH2 in the presence of hydrogen gas due to the increase of ICPMS, heterogeneities have been reported for some sample the equilibrium vapor pressure. -

The Regional Distribution of Zeolites in the Basalts of the Faroe Islands and the Significance of Zeolites As Palaeo- Temperature Indicators
The regional distribution of zeolites in the basalts of the Faroe Islands and the significance of zeolites as palaeo- temperature indicators Ole Jørgensen The first maps of the regional distribution of zeolites in the Palaeogene basalt plateau of the Faroe Islands are presented. The zeolite zones (thomsonite-chabazite, analcite, mesolite, stilbite-heulandite, laumontite) continue below sea level and reach a depth of 2200 m in the Lopra-1/1A well. Below this level, a high temperature zone occurs characterised by prehnite and pumpellyite. The stilbite-heulan- dite zone is the dominant mineral zone on the northern island, Vágar, the analcite and mesolite zones are the dominant ones on the southern islands of Sandoy and Suðuroy and the thomsonite-chabazite zone is dominant on the two northeastern islands of Viðoy and Borðoy. It is estimated that zeolitisa- tion of the basalts took place at temperatures between about 40°C and 230°C. Palaeogeothermal gradients are estimated to have been 66 ± 9°C/km in the lower basalt formation of the Lopra area of Suðuroy, the southernmost island, 63 ± 8°C/km in the middle basalt formation on the northernmost island of Vágar and 56 ± 7°C/km in the upper basalt formation on the central island of Sandoy. A linear extrapolation of the gradient from the Lopra area places the palaeosurface of the basalt plateau near to the top of the lower basalt formation. On Vágar, the palaeosurface was somewhere between 1700 m and 2020 m above the lower formation while the palaeosurface on Sandoy was between 1550 m and 1924 m above the base of the upper formation. -

Zeolite Application in Crop Production: Importance to Soil Nutrient, Soil Water, Soil Health, and Environmental Pollution Management Isaac K
az1851 September 2020 Zeolite Application in Crop Production: Importance to Soil Nutrient, Soil Water, Soil Health, and Environmental Pollution Management Isaac K. Mpanga, Hattie Braun and James Walworth Introduction Benefits of zeolites as a soil conditioner According to the United States Geological Service (USGS), and growth media zeolites (Fig. 1) are hydrated aluminosilicates of alkaline Zeolite soil conditioners or amendments may provide and alkaline-earth metals with very porous structure. many benefits such as improved water infiltration and Zeolites are used as pet litter, in animal feed, in wastewater reduced surface water run-off and nutrient leaching (Fig. 2). treatment, and as soil amendments. Identified about 200 Benefits from zeolite application could translate to increased years ago, there are over 40 different types that include yield, more efficient use of nutrient inputs, and reduction in analcime, chabazite, clinoptilolite, erionite, ferrierite, environmental pollution of nitrogen (N) and phosphorus heulandite, laumontite, mordenite, and phillipsite. Those (P) in bodies of water, as well as reduction in greenhouse commonly used in crop production as soil conditioners are gas emissions (Fig. 2). The high cation exchange capacity clinoptilolite, erionite, and mordenite. Zeolites are mostly (CEC) and porosity of zeolites allows them to absorb and present in soils and sediments, with clinoptilolite as the trap greenhouse gases such as methane (CH4) and carbon most abundant type. In 2019, the United States had nine dioxide (CO2). zeolite mines producing an estimated 98,000 tons of natural zeolites, a 14% increase from 2018. Chabazite is mined in Effects of zeolites on soil physical properties and Arizona (Hyde, 1982; Flanagan, 2020) and clinoptilolite soil water holding capacity in New Mexico (Hyde, 1982). -

Synthesis and Characterization of Zeolites from Sodium Aluminosilicate Solution
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Repository@USM SYNTHESIS AND CHARACTERIZATION OF ZEOLITES FROM SODIUM ALUMINOSILICATE SOLUTION by SITI AIDA BINTI IBRAHIM Thesis submitted in fulfillment of the requirements for the degree of Master of Science August 2007 ACKNOWLEDGEMENTS Thank you to Allah for His Blessing and giving me an ability and strength to complete this thesis. Firstly, I would like to express my deepest gratitude to my supervisor Dr. Norlia Baharun for her expert guidance, constant attention, valuable suggestions, enthusiastic support and personal concern during the research and through the course of my study. Her fruitful ideas throughout the research project has helped me accomplished this work successfully. Special thanks to the Dean of School of Material and Mineral Resources Engineering, Associate Professor Dr. Khairun Azizi Azizli for her permission to let me use all the brilliant facilities and equipment in completing my project. Under her leadership, she has created a healthy learning environment in the school. I would also like to extend my sincere appreciation for all the lecturers in the school especially to Associate Professor Dr. Azizan Aziz for his support and guidance throughout my study. I would like to thank the technical staffs of School of Materials and Mineral Resources Engineering, especially to Mdm. Fong Lee Lee, Mr. Shaarani, Miss Mahani and Mr. Hasnor for their various contributions in one way or another. To my dear friends, Nurul’Ain, Norwanis, Roshazita , Faizul, Shahrizam, Suhaina, Dr. Hasmaliza, Dr. Zuhailawati and all close members of postgraduate room of School of Materials and Mineral Resources Engineering, thank you for making my life in USM so colorful and enjoyable. -

Valid Unnamed Minerals, Update 2012-01
VALID UNNAMED MINERALS, UPDATE 2012-01 IMA Subcommittee on Unnamed Minerals: Jim Ferraiolo*, Jeffrey de Fourestier**, Dorian Smith*** (Chairman) *[email protected] **[email protected] ***[email protected] Users making reference to this compilation should refer to the primary source (Dorian G.W. Smith & Ernest H. Nickel (2007): A System of Codification for Unnamed Minerals: Report of the SubCommittee for Unnamed Minerals of the IMA Commission on New Minerals, Nomenclature and Classification. Canadian Mineralogist v. 45, p.983-1055), and to this website . Additions and changes to the original publication are shown in blue print; deletions are "greyed out and struck through". IMA Code Primary Reference Secondary Comments Reference UM1886-01-OC:HNNa *Bull. Soc. Minéral. 9 , 51 Dana (7th) 2 , 1104 Probably an oxalate but if not is otherwise similar to lecontite UM1892-01-F:CaY *Am. J. Sci. 44 , 386 Dana (7th) 2 , 37 Low analytical total because F not reported; unlike any other known fluoride 3+ UM1910-01-PO:CaFeMg US Geol. Surv. Bull. 419, 1 Am. Mineral. 34 , 513 (Ca,Fe,Mg)Fe 2(PO4)2(OH)2•2H2O; some similarities to mitridatite UM1913-01-AsO:CaCuV *Am. J. Sci. 35 , 441 Dana (7th) 2 , 818 Possibly As-bearing calciovolborthite UM1922-01-O:CuHUV *Izv. Ross. Akad. Nauk [6], 16 , 505 Dana (7th) 2 , 1048 Some similarities to sengierite 3+ UM1926-01-O:HNbTaTiU *Bol. Inst. Brasil Sc., 2 , 56 Dana (7th) 1 , 807 (Y,Er,U,Th,Fe )3(Ti,Nb,Ta)10O26; some similarities to samarskite-(Y) UM1927-01-O:CaTaTiW *Gornyi Zhurn. -

ZEOLITES by Robert L
ZEOLITES By Robert L. Virta Domestic survey data and table were prepared by Christopher H. Lindsay, statistical assistant. Zeolites are hydrated aluminosilicates of the alkaline and The company reported sales of 16,000 t of clinoptilolite in alkaline-earth metals. About 40 natural zeolites have been 2001. Markets for their clinoptilolite products include identified during the past 200 years; the most common are absorbents, air and water filtration, animal feed supplements, analcime, chabazite, clinoptilolite, erionite, ferrierite, environmental products, odor control, and soil conditioners heulandite, laumontite, mordenite, and phillipsite. More than (Goldfield Corp., 2002a, p. 4-5; 2002b). 150 zeolites have been synthesized; the most common are United States Antimony Corp. (USAC) began mining a zeolites A, X, Y, and ZMS-5. Natural and synthetic zeolites are zeolite deposit near Preston, ID. The deposit is 245 meters used commercially because of their unique adsorption, thick and contains reserves exceeding 220 million metric tons ion-exchange, molecular sieve, and catalytic properties. (Mt) of clinoptilolite. Bear River Zeolite, LLC (which is 75% owned by USAC), will operate the mine and mill. The Natural Zeolites company is anticipating production capacity of 200 metric tons per day. The company plans to sell zeolite as an anticaking Commercial zeolite deposits in the United States are agent, feed additive, oil absorbent, soil amendment, and for associated with the alteration of volcanic tuffs in alkaline lake water filtration. Bear River Zeolite also is developing a deposits and open hydrologic systems. Commercial deposits in fertilizer product consisting of ammoniated zeolite and the United States are in Arizona, California, Idaho, Nevada, phosphate for use on fruit, vegetable, and potato crops and in New Mexico, Oregon, Texas, Utah, and Wyoming. -

Zeolite Synthesis Introduction Silicate Minerals Make up a Majority
Created by Erica Gunn, Simmons College ([email protected]) and posted on VIPEr (www.ionicviper.org ) on January 6, 2015. Copyright Erica Gunn 2015. This work is licensed under the Creative Commons Attribution-NonCommerical-ShareAlike 3.0 Unported License. To view a copy of this license visit http://creativecommons.org/about/license/ . Zeolite Synthesis Introduction 1,2 Silicate minerals make up a majority (more than 90%) of the earth’s crust, and approximately 30% of all classified mineral structures. This incredibly diverse group of minerals is based on a 4- basic bonding unit composed of silicon bound to oxygen to form a tetrahedral SiO 4 ion. The simplest silicate is the SiO 2 (silica) structure, where each oxygen at the corner of a tetrahedron is shared with two silicon atoms, giving the compound an overall ratio of 1Si : 2O. When a single oxygen atom participates in two or more tetrahedra, this creates chains, sheets, or other complicated 3-D structures of interlinked silicate ions. The silicate minerals are formed by reaction of acidic silica with basic metal oxides, and are classified according to the arrangement of silicon tetrahedra within the resulting crystal structures. It is possible for other metals to substitute for the silicon atom in some of the silicate tetrahedra. One common substitution is that of Al 3+ for Si 4+ . The ionic radii of these two metals are similar (67 and 54 pm, respectively), so the aluminum is able to fit into the space that is usually occupied by the Si atom. However, the charges on the two metals are different. -

Properties of Concrete 2 Reports
HIGHWAY RESEARCH RECORD Number 43 Properties of Concrete 2 Reports Presented at the 42nd ANNUAL MEETING January 7-11, 1963 HIGHWAY RESEARCH BOARD of the Di vision of Engineering and Industrial Research National Academy of Sciences National Research Council Washington, D. C. 1963 Department of Materials and Construction John H. Swanberg, Chairman Chief Engineer, Minnesota Department of Highways, St. Paul CONCRETE DIVISION Bryant Mather, Chairman Engineer, Concrete Division, Waterways Experiment Station Jackson, Mississippi Harry H. McLean, Vice-Chairman Director, Materials Laboratory New York State Department of Public Works, Albany COMMITTEE ON PERFORMANCE OF CONCRETE-CHEMICAL ASPECTS Howard Newlon, Jr., Chairman Highway Research Engineer Virginia Council of Highway Investigation and Research Charlottesville W. F. Abercrombie, State Highway Materials Engineer, State Highway Department of Georgia, Atlanta E. 0. Axon, Chief of Research, Division of Materials, Missouri State Highway Depart- ment, Jefferson City W. L. Dolch, Joint Highway Research Project, Purdue University, Lafayette, Indiana W. E. Gibson, Engineer of Tests, State Highway Commission of Kansas, Manhattan W. J. Halstead, U.S. Bureau of Public Roads, Washington, D. C. W. C. Hansen, Consulting Chemist, Valparaiso, Indiana G. M. ldorn, Allborg Portland-Cement Fabrik, Concrete Research Laboratory, Copenhagen-Naerum, Denmark John Lemish, Department of Geology, Iowa State University, Ames Kathariue rv1ather, \Vater-ways Experinient Station., Jackson, lVIiss iss ippi Phillip L. Melville, Civil Engineering Branch, Engineering Division, Military Construc tion, Office of Chief of Engineers, Department of the Army, Washington, D. C. Richard C. Mielenz, Director of Research, The Master Builders Company, Cleveland, Ohio C. C. Oleson, Principal Research Engineer, Portland Cement Association, Skokie, Illinois Robert G. -

Geomicrobiology
Newsletter of the Mineralogical Society of America Vol. 13, No.2, May 1997 • ICe GEOMICROBIOLOGY: INTERACTIONS BETWEEN MICROBES AND MINERALS The mineralogical and geochemical characteristics Topics to be covered in the short course include the of the crust, hydrosphere, and atmosphere have evolved links between evolution of the Earth and in parallel with evolution of microorganisms. microorganisms, new insights into microbial diversity in Microorganisms cause mineral precipitation and natural environments and the nature of early life forms, dissolution and control the distribution of elements in the structure, chemistry, and reactivity of mineral diverse environments at and below the surface of the surfaces, the metabolic roles of metals and mechanisms Earth. Conversely, mineralogical and geochemical used to modulate metal concentrations, microbial factors exert important controls on microbial evolution attachment to mineral surfaces and biofilm formation, and the structure of microbial communities. microbial precipitation and the characteristics of The Mineralogical Society of America is offering a biologically precipitated minerals, microbially-mediated Short Course on Geomicrobiology on October 18-19, mineral dissolution, and the impacts of microbial 1997 in Alta, Utah. This course precedes the processes on geochemical cycles in soils and sediments. Mineralogical Society of America - Geological Society Convenors of the Short Course are Jillian F. America Annual Meeting in Salt Lake City, Utah. Banfield (Department of Geology and Geophysics, The short course will teach geologists about the ways in University of Wisconsin-Madison) and Kenneth which microbial activity impacts what have traditionally Nealson (University of Wisconsin Great Lakes Institute, been considered inorganic processes and microbiologists University of Wisconsin - Milwaukee). Additional and other life scientists about the mineralogical and information and registration materials are included in geochemical aspects of biogeochemical systems. -

Absorbing Ammonia
Absorbing Ammonia Introduction A zeolite is a type of mineral that is made up of a framework of silicon, aluminum, and oxygen. This framework acts as a cage and traps other molecules inside of the zeolite. There are many types of zeolites, and each type has a different type of cage structure. The different types of cages will trap different types of molecules. In this activity, you will be working with a natural zeolite (one that is mined out of the ground instead of produced in a lab) called clinoptilolite. This zeolite is often used as an ammonia absorber, meaning the cages in the zeolite trap ammonia molecules. If you have a fish tank with a filter, you have probably seen clinoptilolite—it looks like small white rocks which go into the filter. (It is also sometimes mixed with black rocks called activated carbon which also filter water.) Clinoptilolite is used in fish tanks to remove ammonia because ammonia can damage the fishes’ gills. In this activity, you will see how clinoptilolite can take ammonia out of water, and you can experiment if clinoptilolite will remove anything else from water. In this activity we will: • Use a natural zeolite called clinoptilolite to absorb (take out) ammonia from distilled water. You can also experiment with other materials to see if the zeolite will absorb them too. Materials • Zeolite granules which can be purchased from a store that sells fish supplies. The zeolite can be found in the filter section under ammonia filters. • Disposable cups (12 – 16 oz or larger) • Coffee filter (white paper can be used) • Water • Ammonia • Disposable spoons • Pen or pencil • Scissors Safety Parents, please note that ammonia can be very dangerous! Ammonia can be very dangerous to one’s eyes or if it is swallowed. -
Zeolites Data Sheet
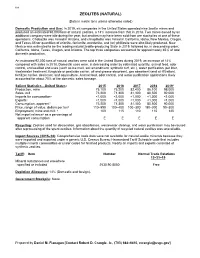
188 ZEOLITES (NATURAL) (Data in metric tons unless otherwise noted) Domestic Production and Use: In 2019, six companies in the United States operated nine zeolite mines and produced an estimated 98,000 tons of natural zeolites, a 14% increase from that in 2018. Two mines owned by an additional company were idle during the year, but zeolites may have been sold from ore stockpiles at one of these operations. Chabazite was mined in Arizona, and clinoptilolite was mined in California, Idaho, New Mexico, Oregon, and Texas. Minor quantities of erionite, ferrierrite, mordenite, and (or) phillipsite were also likely produced. New Mexico was estimated to be the leading natural zeolite-producing State in 2019, followed by, in descending order, California, Idaho, Texas, Oregon, and Arizona. The top three companies accounted for approximately 85% of total domestic production. An estimated 92,000 tons of natural zeolites were sold in the United States during 2019, an increase of 14% compared with sales in 2018. Domestic uses were, in decreasing order by estimated quantity, animal feed, odor control, unclassified end uses (such as ice melt, soil amendment, synthetic turf, etc.), water purification, pet litter, wastewater treatment, fungicide or pesticide carrier, oil and grease absorbent, gas absorbent (and air filtration), fertilizer carrier, desiccant, and aquaculture. Animal feed, odor control, and water purification applications likely accounted for about 70% of the domestic sales tonnage. Salient Statistics—United States: 2015 2016 2017 2018 2019e -

Tech Bulletin
United States Office of Air Quality EPA 456/F-98-004 Environmental Protection Planning and Standards July 1998 Agency Research Triangle Park, NC 27711 Air THIS PAGE INTENTIALLY LEFT BLANK EPA-456/F-98-004 July 1998 ZEOLITE A VERSATILE AIR POLLUTANT ADSORBER Prepared by Clean Air Technology Center (MD-12) Information Transfer and Program Integration Division Office of Air Quality Planning and Standards U.S. Environmental Protection Agency Research Triangle Park, North Carolina 27711 DISCLAIMER This report has been reviewed by the Information Transfer and Program Integration Division of the Office of Air Quality Planning and Standards, U.S. Environmental Protection Agency and approved for publication. Approval does not signify that the contents of this report reflect the views and policies of the U.S. Environmental Protection Agency. Mention of trade names or commercial products is not intended to constitute endorsement or recommendation for use. Copies of this report are available form the National Technical Information Service, U.S. Department of Commerce, 5285 Port Royal Road, Springfield, Virginia 22161, telephone number (800) 553-6847. iv FORWARD The Clean Air Technology Center (CATC) serves as a resource on all areas of emerging and existing air pollution prevention and control technologies, and provides public access to data and information on their use, effectiveness and cost. In addition, the CATC will provide technical support, including access to EPA’s knowledge base, to government agencies and others, as resources allow, related