Bitter Orange) Extract and Its Primary Protoalkaloid P-Synephrine Sidney J
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cinnamon Kumquats
Preserve Today, Relish Tomorrow UCCE Master Food Preservers of El Dorado County 311 Fair Lane, Placerville CA 95667 Helpline (530) 621-5506 • Email: [email protected] • Visit us on Facebook and Twitter! Cinnamon Kumquats “How about a kumquat, my little chickadee?” (W.C. Fields, My Little Chickadee,1940) Say what? Yes, I said kumquats. Those adorable little kumquats. You know, those “things” that you have been so curious about. Another idea for using citrus that is not a marmalade. Vive la différence! That said, a kumquat marmalade is nothing short of marvelous. Honestly. “A kumquat is not an orange though it wants to be one, especially when they’re around other kumquats. (W.C. Fields, It’s A Gift, 1934) Kumquats are native to China, and their name comes from the Cantonese kam kwat, which means "golden orange." They are a symbol of prosperity and a traditional gift at Lunar New Year. Unlike other citrus, kumquats are eaten whole, including the skin. They have a tart-bitter-sweet taste that is boldly refreshing. Ya gotta try one. Really. Just pop one in your mouth and go for it. Fresh kumquats are wonderful in salads and in savory dishes. They are also great in chutneys and relishes. We canned them in a sweet cinnamon syrup. They can then be eaten right out of the jar like candy or used in desserts such as pound cakes or cheesecakes. The syrup is wonderful for drizzles, too. Savory ideas: use them in salads (use the syrup in your dressing!), they would be perfect with ham, maybe as a glaze for chicken wings (I would add some hot sauce, too). -

Tropical Horticulture: Lecture 32 1
Tropical Horticulture: Lecture 32 Lecture 32 Citrus Citrus: Citrus spp., Rutaceae Citrus are subtropical, evergreen plants originating in southeast Asia and the Malay archipelago but the precise origins are obscure. There are about 1600 species in the subfamily Aurantioideae. The tribe Citreae has 13 genera, most of which are graft and cross compatible with the genus Citrus. There are some tropical species (pomelo). All Citrus combined are the most important fruit crop next to grape. 1 Tropical Horticulture: Lecture 32 The common features are a superior ovary on a raised disc, transparent (pellucid) dots on leaves, and the presence of aromatic oils in leaves and fruits. Citrus has increased in importance in the United States with the development of frozen concentrate which is much superior to canned citrus juice. Per-capita consumption in the US is extremely high. Citrus mitis (calamondin), a miniature orange, is widely grown as an ornamental house pot plant. History Citrus is first mentioned in Chinese literature in 2200 BCE. First citrus in Europe seems to have been the citron, a fruit which has religious significance in Jewish festivals. Mentioned in 310 BCE by Theophrastus. Lemons and limes and sour orange may have been mutations of the citron. The Romans grew sour orange and lemons in 50–100 CE; the first mention of sweet orange in Europe was made in 1400. Columbus brought citrus on his second voyage in 1493 and the first plantation started in Haiti. In 1565 the first citrus was brought to the US in Saint Augustine. 2 Tropical Horticulture: Lecture 32 Taxonomy Citrus classification based on morphology of mature fruit (e.g. -

Citrus Trifoliata (Rutaceae): Review of Biology and Distribution in the USA
Nesom, G.L. 2014. Citrus trifoliata (Rutaceae): Review of biology and distribution in the USA. Phytoneuron 2014-46: 1–14. Published 1 May 2014. ISSN 2153 733X CITRUS TRIFOLIATA (RUTACEAE): REVIEW OF BIOLOGY AND DISTRIBUTION IN THE USA GUY L. NESOM 2925 Hartwood Drive Fort Worth, Texas 76109 www.guynesom.com ABSTRACT Citrus trifoliata (aka Poncirus trifoliata , trifoliate orange) has become an aggressive colonizer in the southeastern USA, spreading from plantings as a horticultural novelty and use as a hedge. Its currently known naturalized distribution apparently has resulted from many independent introductions from widely dispersed plantings. Seed set is primarily apomictic and the plants are successful in a variety of habitats, in ruderal habits and disturbed communities as well as in intact natural communities from closed canopy bottomlands to open, upland woods. Trifoliate orange is native to southeastern China and Korea. It was introduced into the USA in the early 1800's but apparently was not widely planted until the late 1800's and early 1900's and was not documented as naturalizing until about 1910. Citrus trifoliata L. (trifoliate orange, hardy orange, Chinese bitter orange, mock orange, winter hardy bitter lemon, Japanese bitter lemon) is a deciduous shrub or small tree relatively common in the southeastern USA. The species is native to eastern Asia and has become naturalized in the USA in many habitats, including ruderal sites as well as intact natural commmunities. It has often been grown as a dense hedge and as a horticultural curiosity because of its green stems and stout green thorns (stipular spines), large, white, fragrant flowers, and often prolific production of persistent, golf-ball sized orange fruits that mature in September and October. -

Facts About Citrus Fruits and Juices: Grapefruit1 Gail C
Archival copy: for current recommendations see http://edis.ifas.ufl.edu or your local extension office. FSHN02-6 Facts About Citrus Fruits and Juices: Grapefruit1 Gail C. Rampersaud2 Grapefruit is a medium- to large-sized citrus fruit. It is larger than most oranges and the fruit may be flattened at both ends. The skin is mostly yellow but may include shades of green, white, or pink. Skin color is not a sign of ripeness. Grapefruit are fully ripe when picked. Popular varieties of Florida grapefruit include: Did you know… Marsh White - white to amber colored flesh and almost seedless. Grapefruit was first Ruby Red - pink to reddish colored flesh with few seeds. discovered in the West Flame - red flesh and mostly seedless. Indies and introduced to Florida in the 1820s. Most grapefruit in the U.S. is still grown in Florida. Compared to most citrus fruits, grapefruit have an extended growing season and several Florida Grapefruit got its name because it grows in varieties grow from September through June. clusters on the tree, just like grapes! Fresh citrus can be stored in any cool, dry place but will last longer if stored in the refrigerator. Do Imposter!! not store fresh grapefruit in plastic bags or film- wrapped trays since this may cause mold to grow on the fruit. Whether you choose white or pink grapefruit or grapefruit juice, you’ll get great taste and a variety of health benefits! Read on…. 1. This document is FSHN026, one of a series of the Food Science and Human Nutrition Department, Florida Cooperative Extension Service, Institute of Food and Agricultural Sciences, University of Florida. -
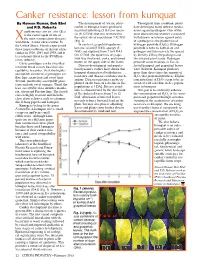
Canker Resistance: Lesson from Kumquat by Naveen Kumar, Bob Ebel the Development of Asiatic Citrus Throughout Their Evolution, Plants and P.D
Canker resistance: lesson from kumquat By Naveen Kumar, Bob Ebel The development of Asiatic citrus Throughout their evolution, plants and P.D. Roberts canker in kumquat leaves produced have developed many defense mecha- anthomonas citri pv. citri (Xcc) localized yellowing (5 DAI) or necro- nisms against pathogens. One of the is the causal agent of one of sis (9-12 DAI) that was restricted to most characteristic features associated the most serious citrus diseases the actual site of inoculation 7-12 DAI with disease resistance against entry X (Fig. 2). of a pathogen is the production of worldwide, Asiatic citrus canker. In the United States, Florida experienced In contrast, grapefruit epidermis hydrogen peroxide (H2O2). Hydrogen three major outbreaks of Asiatic citrus became raised (5 DAI), spongy (5 peroxide is toxic to both plant and canker in 1910, 1984 and 1995, and it DAI) and ruptured from 7 to 8 DAI. pathogen and thus restricts the spread is a constant threat to the $9 billion On 12 DAI, the epidermis of grape- by directly killing the pathogen and citrus industry. fruit was thickened, corky, and turned the infected plant tissue. Hydrogen Citrus genotypes can be classified brown on the upper side of the leaves. peroxide concentrations in Xcc-in- into four broad classes based on sus- Disease development and popula- fected kumquat and grapefruit leaves ceptibility to canker. First, the highly- tion dynamics studies have shown that were different. Kumquat produces susceptible commercial genotypes are kumquat demonstrated both disease more than three times the amount of Key lime, grapefruit and sweet lime. -

The Asian Citrus Psyllid and the Citrus Disease Huanglongbing
TheThe AsianAsian CitrusCitrus PsyllidPsyllid andand thethe CitrusCitrus DiseaseDisease HuanglongbingHuanglongbing Psyllid Huanglongbing The psyllid (pronounced síl - lid) is a small insect, about the size of an aphid The pest insect It has an egg stage, 5 wingless intermediate stages called nymphs, and winged adults Adult The pest insect Egg 5 Nymphs (insects molt to grow bigger) Adult psyllids usually feed on the underside of leaves and can feed on either young or mature leaves. This allows adults to survive year -round. The pest insect When feeding, the adult leans forward on its elbows and tips its rear end up in a very characteristic 45 o angle. The eggs are yellow -orange, tucked into the tips of tiny new leaves, and they are difficult to see because they are so small The pest insect The nymphs produce waxy tubules that direct the honeydew away from their bodies. These waxy tubules are unique and easy to recognize. Nymphs can only survive by living on young, tender The leaves and stems. pest insect Thus, nymphs are found only when the plant is producing new leaves. As Asian citrus psyllid feeds, it injects a salivary toxin that causes the tips of new leaves to easily break off. If the leaf survives, then it twists as it grows. Twisted leaves can be a sign that the psyllid has been there. The pest insect What plants can the psyllid attack? All types of citrus and closely related plants in the Rutaceae family • Citrus (limes, lemons, oranges, grapefruit, mandarins…) • Fortunella (kumquats) • Citropsis (cherry orange) • Murraya paniculata (orange jasmine) • Bergera koenigii (Indian curry leaf) • Severinia buxifolia (Chinese box orange) Plants • Triphasia trifolia (limeberry) • Clausena indica (wampei) affected • Microcitrus papuana (desert-lime) • Others…. -

Citrus Fruits 2020 Summary (August 2020) 3 USDA, National Agricultural Statistics Service
United States Department of Citrus Fruits Agriculture National 2020 Summary Agricultural Statistics Service August 2020 ISSN: 1948-9048 Contents Utilized Citrus Production – United States Chart ................................................................................................................... 6 Citrus Value of Production – United States Chart .................................................................................................................. 6 Citrus Narrative ....................................................................................................................................................................... 7 Citrus Acreage, Production, Utilization, and Value – States and United States: 2017-2018, 2018-2019, and 2019-2020 ........................................................................................................................................................................ 8 Citrus Acreage, Production, Utilization, and Value by Crop – United States: 2017-2018, 2018-2019, and 2019-2020 ........................................................................................................................................................................ 9 Orange Acreage, Yield, Utilization, Price, and Value by Type – States and United States: 2017-2018, 2018-2019, and 2019-2020 ................................................................................................................................................... 10 Bearing Acres of Oranges – United States Chart ................................................................................................................. -

FEMA GRAS Assessment of Natural Flavor Complexes Citrus-Derived
Food and Chemical Toxicology 124 (2019) 192–218 Contents lists available at ScienceDirect Food and Chemical Toxicology journal homepage: www.elsevier.com/locate/foodchemtox FEMA GRAS assessment of natural flavor complexes: Citrus-derived T flavoring ingredients Samuel M. Cohena, Gerhard Eisenbrandb, Shoji Fukushimac, Nigel J. Gooderhamd, F. Peter Guengeriche, Stephen S. Hechtf, Ivonne M.C.M. Rietjensg, Maria Bastakih, ∗ Jeanne M. Davidsenh, Christie L. Harmanh, Margaret McGowenh, Sean V. Taylori, a Havlik-Wall Professor of Oncology, Dept. of Pathology and Microbiology, University of Nebraska Medical Center, 983135 Nebraska Medical Center, Omaha, NE, 68198- 3135, USA b Food Chemistry & Toxicology, Kühler Grund 48/1, 69126 Heidelberg, Germany c Japan Bioassay Research Center, 2445 Hirasawa, Hadano, Kanagawa, 257-0015, Japan d Dept. of Surgery and Cancer, Imperial College London, Sir Alexander Fleming Building, London, SW7 2AZ, United Kingdom e Dept. of Biochemistry, Vanderbilt University School of Medicine, Nashville, TN, 37232-0146, USA f Masonic Cancer Center, Dept. of Laboratory Medicine and Pathology, University of Minnesota, Cancer and Cardiovascular Research Building, 2231 6th St. SE, Minneapolis, MN, 55455, USA g Division of Toxicology, Wageningen University, Stippeneng 4, 6708 WE, Wageningen, the Netherlands h Flavor and Extract Manufacturers Association, 1101 17th Street, NW Suite 700, Washington, DC, 20036, USA i Scientific Secretary to the FEMA Expert Panel, 1101 17th Street, NW Suite 700, Washington, DC,20036,USA ARTICLE INFO ABSTRACT Keywords: In 2015, the Expert Panel of the Flavor and Extract Manufacturers Association (FEMA) initiated a re-evaluation Citrus of the safety of over 250 natural flavor complexes (NFCs) used as flavoring ingredients. This publication isthe Natural flavor complex first in a series and summarizes the evaluation of54 Citrus-derived NFCs using the procedure outlined in Smith Botanical et al. -

A Mandarin by Any Other Name
A mandarin by any other name Page 1 a Mandarin Publication Number 31-111 By Any Other Name (published Dec.2004) Author: Cindy Fake, Horticulture and Small Farms Advisor, Placer and Nevada Counties A mandarin by any other name technically correct. Interestingly, 2. Mediterranean mandarins, would taste as sweet, but what is DNA technology has revealed called “Willowleaf” mandarin it? In Japanese, mandarin is that the common or sweet orange because of its small narrow mikan; in India; it is the suntara. is probably a hybrid of a leaves In French and German, it is pummelo, a large, thick-skinned mandarine; in Italian, mandarino, citrus, and a mandarin. So, even 3. King mandarins, a small Spanish, Portuguese, Romanian, your orange is part mandarin! group of mandarins of Indo- and Bulgarian all use mandarina; China, important primarily as but to many Americans, mandarin Where did tangerine come from? parents of commercial is an unfamiliar term. Mandarins were first imported varieties such as Kinnow and from China into the Encore There is a lot of confusion about Mediterranean region through the mandarins and tangerines. Some port of Tangiers, hence the name 4. Common people say that if the skin is tangerine. However, to quote one mandarins, a reddish-orange and it has seeds, citrus expert, Lance Walheim, diverse group it is a tangerine, and that only “The name tangerine has no that includes Satsumas are mandarins. Others botanical standing; rather it numerous think all of them are tangerines. appears to have developed as a hybrids and many of what Part of the confusion is because marketing term for bright colored some would call tangerines; mandarins make up the largest (reddish-orange) varieties of the Clementines, Dancy, and most varied group of citrus. -

The Asian Citrus Psyllid and the Citrus Disease Huanglongbing
The Asian Citrus Psyllid and the Citrus Disease Huanglongbing Psyllid M. Rogers Beth Grafton-Cardwell Dept of Entomology, UC Riverside and Director Lindcove Research and Extension Center Huanglongbing It has an egg stage, 5 wingless intermediate stages called nymphs, and winged adults Adult The pest insect Egg 5 Nymphs (insects molt to grow bigger) Adult psyllids can feed on either young or mature leaves. This allows adults to survive year-round. The pest insect M. Rogers When feeding, the adult leans forward on its elbows and tips its rear end up in a very o M. Rogers characteristic 45 angle. The eggs are yellow-orange, tucked into the tips of tiny new leaves. They are difficult to see because they are so small The pest insect M. Rogers The nymphs produce waxy tubules that direct the honeydew away from their bodies. These tubules are unique and easy to recognize. Nymphs can only survive by living on young, tender The leaves and stems. pest insect M. Rogers Thus, nymphs are found only when the plant is producing new leaves. M. Rogers As the psyllid feeds, it injects a salivary toxin that causes the tips of new leaves to easily break off. If the leaf survives, then it twists as it grows. Twisted leaves can be a sign that the psyllid has been there. The pest insect M. Rogers M. Rogers M. Rogers What plants can the psyllid attack? All types of citrus and closely related plants in the Rutaceae family • Citrus (limes, lemons, oranges, grapefruit, mandarins…) • Fortunella (kumquats) • Citropsis (cherry orange) • Murraya paniculata (orange jasmine) • Bergera koenigii (Indian curry leaf) Plants • Severinia buxifolia (Chinese box orange) affected • Triphasia trifolia (limeberry) • Clausena indica (wampei) • Microcitrus papuana (desert-lime) • Others…. -

New and Noteworthy Citrus Varieties Presentation
New and Noteworthy Citrus Varieties Citrus species & Citrus Relatives Hundreds of varieties available. CITRON Citrus medica • The citron is believed to be one of the original kinds of citrus. • Trees are small and shrubby with an open growth habit. The new growth and flowers are flushed with purple and the trees are sensitive to frost. • Ethrog or Etrog citron is a variety of citron commonly used in the Jewish Feast of Tabernacles. The flesh is pale yellow and acidic, but not very juicy. The fruits hold well on the tree. The aromatic fruit is considerably larger than a lemon. • The yellow rind is glossy, thick and bumpy. Citron rind is traditionally candied for use in holiday fruitcake. Ethrog or Etrog citron CITRON Citrus medica • Buddha’s Hand or Fingered citron is a unique citrus grown mainly as a curiosity. The six to twelve inch fruits are apically split into a varying number of segments that are reminiscent of a human hand. • The rind is yellow and highly fragrant at maturity. The interior of the fruit is solid rind with no flesh or seeds. • Fingered citron fruits usually mature in late fall to early winter and hold moderately well on the tree, but not as well as other citron varieties. Buddha’s Hand or Fingered citron NAVEL ORANGES Citrus sinensis • ‘Washington navel orange’ is also known • ‘Lane Late Navel’ was the first of a as the Bahia. It was imported into the number of late maturing Australian United States in 1870. navel orange bud sport selections of Washington navel imported into • These exceptionally delicious, seedless, California. -

An Overview of Citrus Aurantium Used in Treatment of Various Diseases
African Journal of Plant Science Vol. 5(7), pp. 390-395, July 2011 Available online at http://www.academicjournals.org/ajps ISSN 1996-0824 ©2011 Academic Journals Review An overview of Citrus aurantium used in treatment of various diseases Jyotsna A. Saonere Suryawanshi Department of Pharmacy, Government Polytechnic, Amravati (M.S. India), India. E-mail: [email protected] Accepted 22 February, 2011 Citrus aurantium (bitter orange) is a plant belonging to the family Rutaceae, The most important biologically active constituents of the C. aurantium fruits are phenethylamine alkaloids octopamine, synephrine, tyramine, N-methyltyramine and hordenine. It is rich in vitamin C, flavonoids and volatile oil. Synephrine is a primary synthesis compound with pharmacological activities such as vasoconstriction, elevation of blood pressure and relaxation of bronchial muscle. whose fruit extracts have been used for the treatment of various diseases such as gastrointestinal disorders, insomnia, head aches, cardiovascular diseases, cancer, antiseptic, anti-oxidant, antispasmodic, aromatic, astringent, carminative, digestive, sedative, stimulant, stomachic and tonic and by research novel use is found in obesity and related risks even life threatening are continuously increasing through out world in all age groups. Many marketed formulations claim to possess antiobesity actions, but still many herbs which have claims to this need to be investigated and their claims to be authenticated. In recent era there is a great thrust on screening of herbal extracts and formulations for antiobesity action. In this article efforts have been taken to discuss the photochemistry, constituents, ethnobotany, pharmacology safety and toxicity of citrus plant. The motto is to discuss C. aurantium here more research attention should be given on this that would increase its use in various chronic and acute diseases Key words: Bitter orange, synephrine, obesity, thermogenesis, ethnobotany.