Development of a Nanobody-Based ELISA for the Detection of the Insecticides Cyantraniliprole and Chlorantraniliprole in Soil and the Vegetable Bok Choy
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
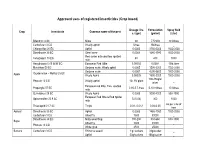
Approved Uses of Registered Insecticides (Crop Based)
Approved uses of registered insecticides (Crop based) Dosage / ha Formulation Spray fluid Crop Insecticide Common name of the pest a.i (gm) (gm/ml) (Liter) Bifenthrin 8 SC Mites 60 7.5ml/lit 10 lit/tree Carbofuran 3 CG Woolly aphid 5/tree 166/tree _ Chlorpyrifos 20 EC Aphid 0.0005 3750-5000 1500-2000 Dimethoate 30 EC Stem borer 0.0003 1485-1980 1500-2000 Red spider mite and two spotted Fenazaquin 10 EC 40 400 1000 mite Hexythiazox 5.45 W/W EC European Red Mite 0.00002 0.0004 10ltr./tree Malathion 50 EC Sanjose scale, Wooly aphid 0.0005 1500-2000 1500-2000 Sanjose scale 0.0007 4200-5600 1500-2000 Oxydemeton – Methyl 25 EC Apple Wooly Aphid 0.00025 1500-2000 1500-2000 100-150gm/ Phorate 10 CG Woolly aphid 10- 15/ plant _ plant European red Mite, Two spotted Propargite 57 EC 2.85-5.7 /tree 5-10 ml/tree 10 lit/tree mite Quinalphos 25 EC Wooly Aphid 0.0005 3000-4000 500-1000 European Red Mite & Red Spider Spiromesifen 22.9 SC 72(0.03) 300 1000 mite As per size of Thiacloprid 21.7 SC Thrips 0.01- 0.012 0.04-0.05 tree Apricot Dimethoate 30 EC Aphid 0.0003 1485-1980 1500-2000 Carbofuran 3 CG Shoot fly 1500 50000 _ Dimethoate 30 EC Milky weed bug 180-200 594-660 500-1000 Bajra Shoot fly 3000 30000 _ Phorate 10 CG White grub 2500 25000 _ Banana Carbofuran 3 CG Rhizome weevil 1 g/ suckers 33g/sucker _ Aphid 50g/suckers 166g/sucker _ Nematode 1.5g/suckers 50g/suckers _ Dimethoate 30 EC Aphid, Lace wing bug 0.0003 1485-1980 1500-2000 Tingyi bug 0.00025 1500-2000 1500-2000 Oxydemeton – Methyl 25 EC Aphids 0.0005 3000-4000 1500-2000 2.5 -1.25/ 25 -12.5/ Phorate 10 CG Aphid _ plant plant Quinalphos 25 EC Tingid bug 0.0005 3000-4000 500-1000 Phosalone 35 EC Aphid 500 1428 500-1000 Aphid 1000 33300 _ Barely Carbofuran 3 CG Jassid 1250 41600 _ Cyst nematode 1000 33300 _ Phorate 10 CG Aphid 1000 10000 _ Beans Chlorpyrifos 20 EC Pod borer , Black bug 600 3000 500-1000 Chlorantraniliprole 18.5 SC Pod borers 25 125 500 Chlorpyrifos 1.5 DP Helicoverpa armigera 375 25000 _ Azadirachtin 0.03 (300 PPM) Pod Borer _ _ _ Bacillus thuringiensis Var. -

Cyantraniliprole
PUBLIC RELEASE SUMMARY on the Evaluation of the New Active Constituent Cyantraniliprole in the Product DuPont Exirel Insecticide APVMA Product Number 64103 OCTOBER 2013 © Australian Pesticides and Veterinary Medicines Authority 2013 ISSN: 1443-1335 (electronic) ISBN: 978-1-922188-50-2 (electronic) Ownership of intellectual property rights in this publication Unless otherwise noted, copyright (and any other intellectual property rights, if any) in this publication is owned by the Australian Pesticides and Veterinary Medicines Authority (APVMA). Creative Commons licence With the exception of the Coat of Arms and other elements specifically identified, this publication is licensed under a Creative Commons Attribution 3.0 Australia Licence. This is a standard form agreement that allows you to copy, distribute, transmit and adapt this publication provided that you attribute the work. A summary of the licence terms is available from www.creativecommons.org/licenses/by/3.0/au/deed.en. The full licence terms are available from www.creativecommons.org/licenses/by/3.0/au/legalcode. The APVMA’s preference is that you attribute this publication (and any approved material sourced from it) using the following wording: Source: licensed from the Australian Pesticides and Veterinary Medicines Authority (APVMA) under a Creative Commons Attribution 3.0 Australia Licence. In referencing this document the Australian Pesticides and Veterinary Medicines Authority should be cited as author, publisher and copyright owner. Use of the Coat of Arms The terms under which the Coat of Arms can be used are set out on the Department of the Prime Minister and Cabinet website (see www.dpmc.gov.au/guidelines). -

CYANTRANILIPROLE (263) the First Draft Was Prepared by Mr. David Lunn New Zealand Food Safety Authority, Wellington, New Zealand
Cyantraniliprole 361 CYANTRANILIPROLE (263) The first draft was prepared by Mr. David Lunn New Zealand Food Safety Authority, Wellington, New Zealand EXPLANATION Cyantraniliprole is a diamide insecticide with a mode of action (ryanodine receptor activation) similar to chlorantraniliprole and flubendiamide. It has root systemic activity with some translaminar movement and is effective against the larval stages of lepidopteran insects; and also on thrips, aphids, and some other chewing and sucking insects. Authorisations exist for the use of cyantraniliprole in Canada, Columbia, Malaysia, New Zealand, Vietnam and the CLISS countries in West Africa. Authorisations are also being progressed in Australia, Europe and USA under an OECD Joint Review exercise. Cyantraniliprole was scheduled by the Forty-fourth Session of the CCPR as a new compound for consideration by the 2013 JMPR. Residue and analytical aspects of cyantraniliprole were considered for the first time by the present meeting. The manufacturer submitted studies on metabolism, analytical methods, supervised field trials, processing, freezer storage stability, environmental fate in soil and rotational crop residues. In this evaluation, the values presented in the tables are as reported in the various studies, but in the accompanying text, they have generally been rounded to two significant digits. Abbreviations have also been used for the various cyantraniliprole metabolites mentioned in the study reports. These include: IN-F6L99 3-Bromo-N-methyl-1H-pyrazole-5-carboxamide IN-HGW87 -

Effects of Cyantraniliprole, a Novel Anthranilic Diamide Insecticide, Against Asian Citrus Psyllid Under Laboratory and Field Co
Submitted to: Correspondence to: Pest Management Science Lukasz L. Stelinski, Entomology and Nematology Department, Citrus Research & Education Center, University of Florida, 700 Experiment Station Road, Lake Alfred, FL 33850. Ph: 863 956 8851. Fax: 863 956 4631. Email: [email protected] Effects of cyantraniliprole, a novel anthranilic diamide insecticide, against Asian citrus psyllid under laboratory and field conditions Siddharth Tiwari and Lukasz L. Stelinski* Entomology and Nematology Department, Citrus Research and Education Center, University of Florida, Lake Alfred, FL 33850 *Corresponding author Accepted Article This article has been accepted for publication and undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process, which may lead to differences between this version and the Version of Record. Please cite this article as doi: 10.1002/ps.3468. © 2012 Society of Chemical Industry Abstract BACKGROUND:The Asian citrus psyllid, Diaphorina citri (Hemiptera: Psyllidae), is the most destructive pest of citrus in Florida. The development of insecticide resistance in several populations of D. citrihas been documented. There is an urgent need to develop and integrate novel tools for the successful management of D. citri and also to prevent the development of insecticide resistance. RESULTS:We investigated the effects of a relatively newer chemistry, cyantraniliprole, against D. citri. The contact toxicity of cyantraniliprole was 297 fold higher against D. citrithan its primary parasitoid,Tamarixia radiata(Hymenoptera: Eulophidae). D. citrisettled and fed less on cyantraniliprole-treated plants than controls at concentrations as low as 0.025 and 0.125 µg AI mL-1, respectively. D. citri egg production, first instar emergence and adult emergence were significantly reduced on plants treated with 0.25, 0.02 and 0.25 µg AI mL-1of cyantraniliprole, respectively, when compared with control plants. -

Possible Enzymatic Mechanism Underlying Chemical Tolerance and Characteristics of Tolerant Population in Scapholeberis Kingi
Possible Enzymatic Mechanism Underlying Chemical Tolerance and Characteristics of Tolerant Population in Scapholeberis Kingi Makoto Ishimota ( [email protected] ) The Institute of Environmental Toxicology https://orcid.org/0000-0003-4686-0244 Mebuki Kodama The Institute of Environmental Toxicology Naruto Tomiyama The Institute of Environmental Toxicology Research Article Keywords: Cladocera, Insecticide, Tolerance, Acetylcholinesterase, Peroxidase, Superoxide dismutase, Multi-generational study, Field population. Posted Date: May 17th, 2021 DOI: https://doi.org/10.21203/rs.3.rs-492302/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Page 1/24 Abstract To determine the potential effects of pesticides on aquatic organisms inhabiting a realistic environment, we explored the characteristics and mechanisms of chemical tolerance in Scapholeberis kingi. We established a chemical-tolerant population via continuous exposure to pirimicarb, an acetylcholinesterase (AChE) inhibitor, and examined the effects of pirimicarb concentration on the intrinsic growth rates (r) of tolerant cladocerans. We also explored the association between r and feeding rate and tested the involvement of antioxidant enzymes [peroxidase (PO) and superoxide dismutase] and AChE in pirimicarb sensitivity. S. kingi was continuously exposed to sublethal pirimicarb concentrations (0, 2.5, 5, and 10 µg/L) for 15 generations and changes (half maximal effective concentration at 48 h, 48 h-EC50) in chemical sensitivity were investigated. In the F14 generation, the sensitivity of the 10 µg/L group was three times lower than that of the control group, suggesting the acquisition of chemical tolerance. Moreover, r was signicantly and negatively correlated with 48 h-EC50, suggesting a tness cost for tolerance. -

Recommended Classification of Pesticides by Hazard and Guidelines to Classification 2019 Theinternational Programme on Chemical Safety (IPCS) Was Established in 1980
The WHO Recommended Classi cation of Pesticides by Hazard and Guidelines to Classi cation 2019 cation Hazard of Pesticides by and Guidelines to Classi The WHO Recommended Classi The WHO Recommended Classi cation of Pesticides by Hazard and Guidelines to Classi cation 2019 The WHO Recommended Classification of Pesticides by Hazard and Guidelines to Classification 2019 TheInternational Programme on Chemical Safety (IPCS) was established in 1980. The overall objectives of the IPCS are to establish the scientific basis for assessment of the risk to human health and the environment from exposure to chemicals, through international peer review processes, as a prerequisite for the promotion of chemical safety, and to provide technical assistance in strengthening national capacities for the sound management of chemicals. This publication was developed in the IOMC context. The contents do not necessarily reflect the views or stated policies of individual IOMC Participating Organizations. The Inter-Organization Programme for the Sound Management of Chemicals (IOMC) was established in 1995 following recommendations made by the 1992 UN Conference on Environment and Development to strengthen cooperation and increase international coordination in the field of chemical safety. The Participating Organizations are: FAO, ILO, UNDP, UNEP, UNIDO, UNITAR, WHO, World Bank and OECD. The purpose of the IOMC is to promote coordination of the policies and activities pursued by the Participating Organizations, jointly or separately, to achieve the sound management of chemicals in relation to human health and the environment. WHO recommended classification of pesticides by hazard and guidelines to classification, 2019 edition ISBN 978-92-4-000566-2 (electronic version) ISBN 978-92-4-000567-9 (print version) ISSN 1684-1042 © World Health Organization 2020 Some rights reserved. -

Insecticide Control of Drosophila Suzukii in Commercial Sweet Cherry Crops Under Cladding
insects Article Insecticide Control of Drosophila suzukii in Commercial Sweet Cherry Crops under Cladding Bethan Shaw 1 , Sebastian Hemer 2, Madeleine F. L. Cannon 1, Francesco Rogai 1 and Michelle T. Fountain 1,* 1 Pest and Pathology Ecology Department, NIAB EMR, New Road, East Malling, Kent ME19 6BJ, UK 2 Research and Development Department, Berry Garden Growers Ltd., Tatlingbury Oast, Five Oak Green, Tonbridge, Kent TN12 6RG, UK * Correspondence: [email protected] Received: 31 May 2019; Accepted: 2 July 2019; Published: 4 July 2019 Abstract: Drosophila suzukii Matsumura is a damaging invasive pest of sweet cherry. Using a series of laboratory leaf contact assays, semi-field, and orchard spray programs we aimed to determine the impact of insecticide programs on D. suzukii adult mortality and oviposition in cladding-protected sweet cherry crops. Tests included assessing adult D. suzukii mortality after contact with leaves sprayed either one or two weeks previously and emergence of adults from fruits. Spinosad, lambda-cyhalothrin, acetamiprid, lime, pyrethrin, deltamethrin, and cyantraniliprole all reduced fruit damage up to day 7 after application. Of these active ingredients, only spinosad, lambda-cyhalothrin, and cyantraniliprole gave satisfactory control up to 14 days. There was no significant difference in D. suzukii mortality when exposed to leaves treated either one or two weeks previously with an application of either spinosad, cyantraniliprole, or lambda-cyhalothrin; however, mortality was significantly higher than D. suzukii in contact with untreated leaves. In eight commercial orchards, fortnightly spray applications including spinosad, cyantraniliprole, and lambda-cyhalothrin gave effective control of D. suzukii until harvest with very few damaged fruits. -

The Insecticides Act, 1968 (Act No.46 of 1968)
The Insecticides Act, 1968 (Act No.46 of 1968) An Act to regulate the import, manufactures, sale, transport, distribution and use of insecticides with a view to prevent risk to human beings or animals and for matters connected therewith. [2 nd September 1968] Be it enacted by Parliament in the Nineteenth Year of the Republic of India as follows: 1. Short title, extent and commencement. * a. This Act may be called the Insecticides Act, 1968. b. It extends to the whole of India. c. It shall come into force on such date as the Central Government may, by notification in the official Gazette, appoint and different dates may be appointed for different States and for different provisions of Act. 2. Application of other laws not barred * The provisions of this Act shall be in addition to, and not in derogation of, any other law for the time being in force. 3. Definitions- In this Act, unless the context otherwise requires- a. "animals" means animals useful to human beings and includes fish and fowl, and such kinds of wild life as the Central Government may, by notification in the official Gazette, specify, being kinds which in its opinion, it is desirable to protect or preserve; b. "Board" means the Central Insecticides Board constituted under Sec.4; c. "Central Insecticides Laboratory" means the Central Insecticides Laboratory established, or as the case may be, the institution specified under Sec.16; d. "Import" means bringing into any place within the territories to which this Act extends from a place outside those territories; e. "Insecticide" means- i. -

A Review of Insecticide Classes and Characteristics
Denver, CO | February 5 - 7, 2020 A Review of Insecticide Classes and Characteristics Whitney Cranshaw Colorado State University Common Types of Pesticides (Organisms Controlled) • Herbicides • Insecticides – Higher Plants – Insects • Algacides • Acaricides/ – Algae Miticides& Ticks • Fungicides • Molluscicides – Fungi – Slugs & Snails • Bactericides – Bacteria Classification of Insecticides Mode of Entry Classification of Insecticides Systemic or Not Systemic? Are they capable of moving within the plant? Distribution of C14 labeled Thiamethoxam™ 25WG after a foliar application to cucumber leaves 1 hour after application 8 hour after application 24 hour after application Slide Credit: N. Rechcigl Systemic insecticides applied to leaves Some systemic insecticide can move into plants when sprayed onto leaves. Some systemic insecticides can move into plant when applied to the roots. Most systemic insecticides will appear in highest concentration in the new growth Systemic insecticides applied to soil Systemic Insecticides • Capable of some translocation in plant • Range exists in ability to move in plant – Some limited to translaminar movement – Some broadly distribute in plant (usually to newer growth) • Systemic activity is limited to a small number of insecticides – Most neonicotinoids – Diamides (limited) – Abamectin (translaminar only) Systemic Insecticides • Capable of some translocation in plant • Range exists in ability to move in plant – Some limited to translaminar movement – Some broadly distribute in plant (usually to newer growth) • Systemic activity is limited to a small number of insecticides –Some organophophates –All neonicotinoids –Diamides (limited) –Avermectins (translaminar only) Translaminar movement – Insecticide can move through a leaf (but not necessarily to another leaf) Example: Foliar applications of abamectin (Avid) Essentially all systemic insecticide move primarily in the xylem of the plant. -

Evaluation of Insecticide Efficacy and Insecticide Adaptive Response in Italian Populations of Drosophila Suzukii
Bulletin of Insectology 74 (1): 103-114, 2021 ISSN 1721-8861 eISSN 2283-0332 Evaluation of insecticide efficacy and insecticide adaptive response in Italian populations of Drosophila suzukii Stefano CIVOLANI1,2, Giacomo VACCARI3, Stefano CARUSO3, Luca FINETTI1, Giovanni BERNACCHIA1, Milvia CHICCA1, Stefano CASSANELLI4 1Department of Life Sciences and Biotechnology, University of Ferrara, Italy 2InnovaRicerca s.r.l., Monestirolo, Ferrara, Italy 3Consorzio Fitosanitario di Modena, Italy 4Department of Life Sciences, University of Modena and Reggio Emilia, Reggio Emilia, Italy Abstract Monitoring sensitivity to insecticides is crucial to prevent outbreaks of invasive pests characterized by high reproductive and adap- tive potential such as the Drosophila suzukii (Matsumura) (Diptera Drosophilidae). The aim of the present study was to investigate the possible appearance of resistance to cyantraniliprole, deltamethrin and spinosad. Field trials on commercial sweet cherry or- chards in Northern Italy showed that two out of six strains were not fully controlled using cyantraniliprole and deltamethrin, while spinosad was thoroughly effective. At the bioassay, two populations showed a decrease in deltamethrin and cyantraniliprole sus- ceptibility (LC50 values 12.7-21.0 and 3.4-5.8 times higher than those from the untreated populations, respectively). Biochemical analyses revealed that low resistance to the pesticides was associated with high monooxygenase and carboxylesterase activities (range 2.68-4.37-and 1.97-2.73 times higher than in the wild population). A dose-dependent increase in cytochrome P450 monoox- ygenase Cyp12d1 and ryanodine receptor gene expression was found when a strain with low resistance to cyantraniliprole in field trials was treated with increasing dosages of the diamide in bioassays. -

PDP Pesticide History Years Each Pesticide Was Reported from 1991 - 2020
USDA, AMS, S&T, MPD - Pesticide Data Program (PDP) 2 November 2020 PDP Pesticide History Years each pesticide was reported from 1991 - 2020 Pest Pesticide Name Code Years Pesticide was Reported 1,2,4-Triazole A68 2003 - 2007 1-Naphthol 382 1994 - 2000, 2003 - 2020 2,3,5-Trimethacarb A72 2020 2,4,5-T 312 2001 - 2016 2,4,5-TP AJE 2010 - 2013 2,4-D 026 1992 - 1998, 2002 - 2020 2,4-DB 317 1996 - 1997, 2003 - 2016, 2019 2,4-dimethyl aniline (2,4 DMA) AGQ 2007 - 2008 2,4-dimethylphenyl formamide (2,4-DMPF) AGR 2007 - 2012, 2016 - 2020 2,6-dichlorobenzamide 272 2010, 2017 2,6-DIPN AFZ 2016 - 2020 3,5-Dichloroaniline ABM 2003 3-Hydroxycarbofuran 512 1993 - 2020 3-ketocarbofuran 218 2001 - 2002 4,4-dibromobenzophenone AGS 2007 - 2008 4-Hydroxychlorothalonil ANM 2017 4-Hydroxydiphenylamine B19 1995 - 1997 5-Hydroxythiabendazole B28 1996 - 1997, 2003 - 2020 Abamectin 948 1994 - 1997, 1999, 2012 - 2020 Acephate 204 1991 - 2020 Acequinocyl AKS 2013 - 2015, 2020 Acetamiprid B80 2004 - 2020 Acetochlor 807 2001, 2003 - 2020 Acetochlor ethanesulfonic acid (ESA) ABN 2001 - 2013, 2017 Acetochlor oxanilic acid (OA) ABO 2001, 2003 - 2013 Acibenzolar S methyl B51 2003 - 2020 Acifluorfen 727 2003 - 2007, 2014 - 2017 Aclonifen D58 2017 - 2020 Acrinathrin A03 2012 - 2015, 2017 Afidopyropen F87 2020 Alachlor 227 1997 - 2020 Alachlor ethanesulfonic acid (ESA) ABP 2001 - 2013, 2017 Alachlor oxanilic acid (OA) ABQ 2001 - 2013 Aldicarb 167 1994 - 2020 Aldicarb sulfone 168 1994 - 2020 Aldicarb sulfoxide 169 1993 - 2020 Aldrin 001 1997 - 2020 Allethrin 002 1994, 1999 -

(12) United States Patent (10) Patent No.: US 7.655,597 B1 Sanders (45) Date of Patent: Feb
USOO7655597 B1 (12) United States Patent (10) Patent No.: US 7.655,597 B1 Sanders (45) Date of Patent: Feb. 2, 2010 (54) PESTICIDE COMPOSITIONS INCLUDING 4,867,972 A 9, 1989 Girardeau et al. POLYMERC AIDUVANTS 6,093,679 A 7/2000 AZuma et al. 6,146,652 A 11/2000 Gore et al. 6,515,091 B2 2/2003 Sanders et al. (75) Inventor: John Larry Sanders, Leawood, KS 6,610,282 B1 8, 2003 Ghosh (US) 6,677,399 B2 1/2004 Herbert et al. 6,703,469 B2 3/2004 Sanders et al. (73) Assignee: Specialty Fertilizer Products, LLC, 6,706,666 B2 3/2004 HaSebe et al. Leawood, KS (US) 6,897,184 B2 5/2005 Kurita et al. 7,407,667 B2 8, 2008 Zerrer et al. (*) Notice: Subject to any disclaimer, the term of this 2004/0226331 A1 11/2004 Sanders et al. patent is extended or adjusted under 35 2004/0248741 A1 12/2004 Gotsche et al. U.S.C. 154(b) by 0 days. 2005/00904O2 A1* 4/2005 Dieing et al. ............... 504,361 2008.0167189 A1 7/2008 Oetter et al. (21) Appl. No.: 12/534,481 2008.0171658 A1 7/2008 Dyllick-Brenzinger et al. (22) Filed: Aug. 3, 2009 * cited by examiner Primary Examiner Johann R Richter (51) Int. Cl. Assistant Examiner—Andriae M Holt AOIN 25/00 (2006.01) (74) Attorney, Agent, or Firm Hovey Williams LLP AOIN 57/26 (2006.01) AOIN 25/28 (2006.01) (57) ABSTRACT (52) U.S. Cl. .................... 504/116.1; 504/206; 504/360; 504/361 Pesticidal compositions of improved effectiveness are pro (58) Field of Classification Search ................