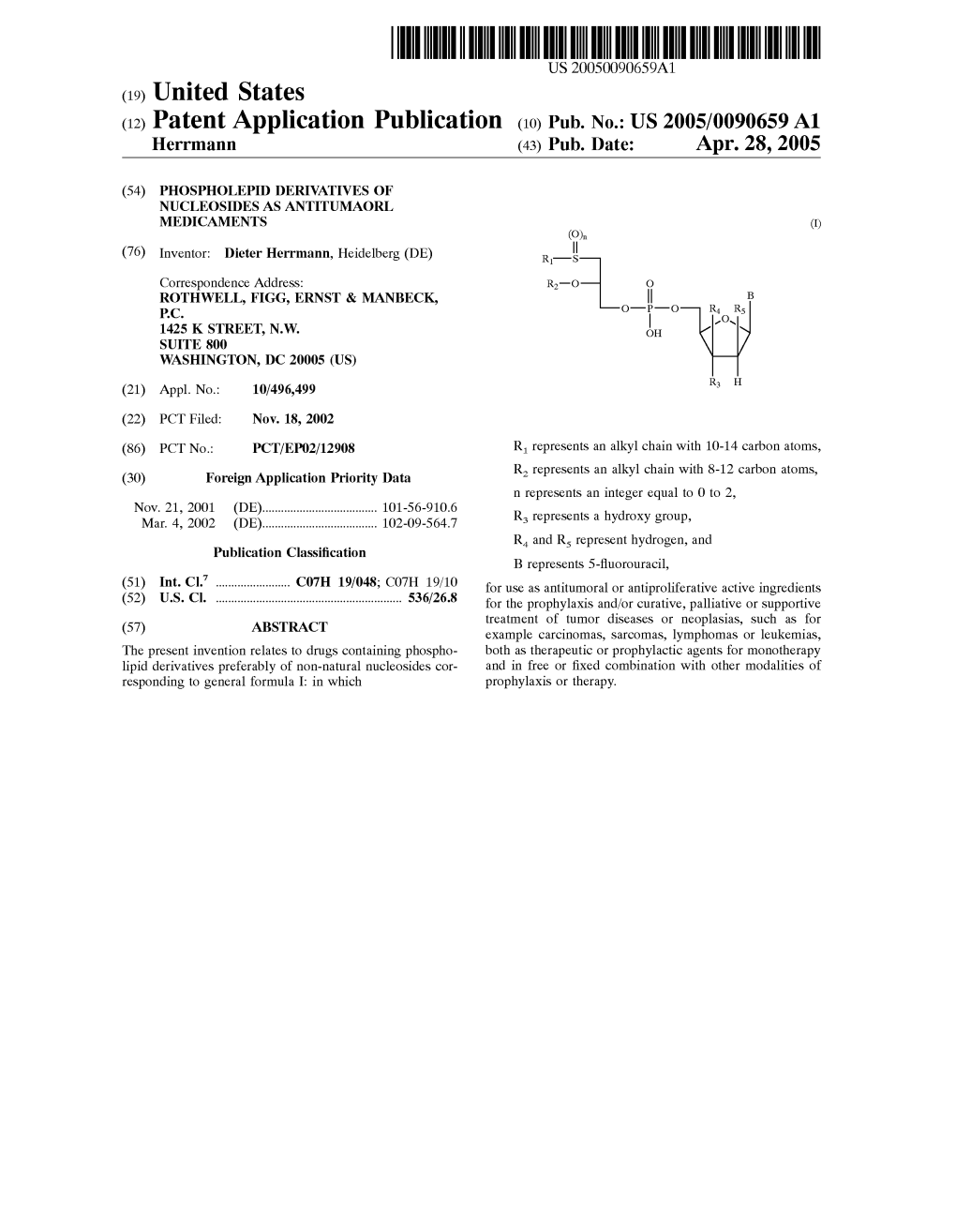
(12) Patent Application Publication (10) Pub
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(12) United States Patent (10) Patent No.: US 8,921,361 B2 Cmiljanovic Et Al
USOO892.1361 B2 (12) United States Patent (10) Patent No.: US 8,921,361 B2 Cmiljanovic et al. (45) Date of Patent: Dec. 30, 2014 (54) TRIAZINE, PYRIMIDINE AND PYRIDINE 409/04 (2013.01); C07D 413/04 (2013.01); ANALOGS AND THEIR USEAS C07D 413/14 (2013.01); C07D 417/04 THERAPEUTICAGENTS AND DAGNOSTIC (2013.01); C07D 417/14 (2013.01); C07D PROBES 491/048 (2013.01); C07D491/147 (2013.01); C07D 495/04 (2013.01); C07D 495/14 (2013.01); C07D498/04 (2013.01); C07D (75) Inventors: Vladimir Cmiljanovic, Basel (CH): 513/04 (2013.01); C07D 519/00 (2013.01) Natasa Cmiljanovic, Basel (CH); Bernd USPC .......................................... 514/232.2:544/83 Giese, Fribourg (CH); Matthias (58) Field of Classification Search Wymann, Bern (CH) None See application file for complete search history. (73) Assignee: University of Basel, Basel (CH) (56) References Cited (*) Notice: Subject to any disclaimer, the term of this patent is extended or adjusted under 35 U.S. PATENT DOCUMENTS U.S.C. 154(b) by 60 days. 5,489,591 A * 2/1996 Kobayashi et al. ........... 514,245 (21) Appl. No.: 13/128,436 7,173,029 B2 2/2007 Hayakawa et al. 8,217,036 B2 * 7/2012 Venkatesan et al. ....... 514,232.2 (22) PCT Filed: Nov. 10, 2009 2010 OO69629 A1 3/2010 Shimma et al. (86). PCT No.: PCT/B2O09/OOT404 FOREIGN PATENT DOCUMENTS EP 1864 665 A1 12/2007 S371 (c)(1), WO 2005/028444 A1 3, 2005 (2), (4) Date: Jul. 1, 2011 WO 2007 1271.75 A2 11/2007 WO 2008/O18426 A1 2, 2008 (87) PCT Pub. -

Mitomycin C in the Treatment of Chronic Myelogenous Leukemia
Nagoya ]. med. Sci. 29: 317-344, 1967. MITOMYCIN C IN THE TREATMENT OF CHRONIC MYELOGENOUS LEUKEMIA AKIRA HosHINo 1st Department of Internal Medicine Nagoya University School oj Medicine (Director: Prof. Susumu Hibino) SUMMARY Studies made of the treatment with 66 courses of mitomycin C in 28 patients with chronic myelogenous leukemia are reported. The effect of mitomycin C was investigated according to the relation between drug and host factors, comparison with the effects of other agents, and drug resistance. Patients with less hematological and clinical symptoms responded better to mitomycin C therapy. The remission rate of cases treated intravenously with mitomycin C was 93.8% and of cases treated orally with mitomycin C was 72.0%. The remission rate of the total cases (intravenous and oral) treated with mitomycin C was 77.3%. The therapeutic effect of mitomycin C is considered to be equal or be somewhat superior to the effect of busulfan as a result of data on the occurrence of resistance, cross resistance, development of acute blastic crisis and life span. Busulfan was effective in patients resistant to mitomycin C, and mitomycin C did not clinically show cross resistance to alkylating agents. Two patients resist· ant to mitomycin C recovered the sensitivity to mitomycin C after treatment with busulfan or 6-mercaptopurine. Side effects were observed in 39.4% of 66 cases, but severe side effect causing suspension of mitomycin C was rare. I. INTRODUCTION Human leukemia serves as a useful investigative model in which the de finite effect of anti-cancer agents can be evaluated quantitatively by factors such as the improvement of hematological findings and clinical symptoms, the remission rate, and the prologation of life span. -

Cross-Linking of DNA by Alkylating Agents and Effects on DNA Function in the Chick Embryo
(CANCER RESEARCH 31, 1573-1579, November 1971] Cross-linking of DNA by Alkylating Agents and Effects on DNA Function in the Chick Embryo Jerome J. McCann, Timothy M. Lo, and D. A. Webster Department of Biology, Illinois Institute of Technology, Chicago, Illinois 60616 SUMMARY single-stranded DNA partitions into the dextran-rich lower phase (3). This assay has been used to demonstrate that many Drug-induced cross-links are found in the DNA of chick preparations of DNA normally contain some cross-linked embryos within 6 hr after injection of mitomycin C and DNA, which was postulated to arise when DNA is sheared methyl-di-(2-chloroethyl)amine into the egg and 24 hr after during purification (2, 3). In Escherichia coli, an enzyme injection of triethylene thiophosphoramide. Effects on the system, consisting of an exonuclease and a ligase, has been rates of synthesis of DNA, RNA, and protein were studied shown to cross-link DNA terminally (25). with chemical assays for total content of these When difunctional alkylating agents were injected onto the macromolecules as well as radioactive precursor incorporation. area vasculosa of 4-day chick embryos in ovo, within 24 hr a All three drugs inhibited DNA synthesis before RNA and specific type of macrophage was formed over the entire protein synthesis were affected, but there were discrepancies embryo (24). These macrophages were indistinguishable by between the two methods of measuring macromolecular light and electron microscopy from macrophages which are synthesis; thus, radioactive precursor incorporation is not formed during the process of "programmed cell death," a always a reliable measurement of macromolecular synthesis in phenomenon believed important for sculpturing the normal the chick embryo. -

Supplementary Table S1 Cell-Based Inhibitory Activity Data of 88
Supplementary Table S1 Cell‐based inhibitory activity data of 88 anticancer drugs. For drugs with multiple cancer cell‐line inhibition data, the best activity is listed. Drug Cell line GI50/IC50 (nM) Reference (Pubmed ID ) 5‐azacytidine NCI‐H460 147.9 20442306 5‐fluorouracil OVCAR‐3 21.4 20442306 6‐Mercaptopurine K‐562 354.8 20442306 Actinomycin D SR 0.0019 20442306 Altretamine HOP‐18 60534.1 NCI standard agent database Anastrozole SK‐MEL‐2 100.0 20442306 Arsenic trioxide CCRF‐CEM 512.9 20442306 Bendamustine MOLT‐4 1148.2 20442306 Bleomycin MALME‐3M 2.9 20442306 Bortezomib RPMI‐8226 0.2 20442306 Busulfan LOX IMVI 6025.6 20442306 Capcitebine SK‐MEL‐2 10.0 20442306 Carboplatin KLE 240.0 8123477 Carmustine SW 1783 1524.1 NCI standard agent database Chlorambucil SR 812.8 20442306 Cisplatin SR 77.6 20442306 Cladribine HL60 8.7 9845378 Clofarabine CCRF‐CEM 10.0 20442306 Cyclophosphamide COLO 746 25.0 NCI standard agent database Cytarabine HCl CCRF‐CEM 6.0 20442306 Dacarbazine HL‐60(TB) 4168.7 20442306 Dasatinib K‐562 10.0 20442306 Daunorubicin MOLT‐4 2.8 20442306 Delta‐1‐testololactone HOP‐19 18793.2 NCI standard agent database Dimethyltestosterone RPMI‐8226 7585.8 20442306 Docetaxel NCI‐H522 0.1 20442306 Doxorubicin MOLT‐4 10.0 20442306 Dromostanolone HOP‐92 407.4 20442306 propionate Epirubicin SR 10.0 20442306 Erlotinib EKVX 53.7 20442306 Estramustine CCRF‐CEM 25.1 20442306 Ethacrynic acid Primary Chronic 8560.0 20011538 Lymphocytic Leukemia cells Ethinyl estradiol U251 7762.5 20442306 Etoposide MOLT‐4 195.0 20442306 Everolimus RPMI‐8226 -

Open Full Page
Published OnlineFirst November 14, 2011; DOI: 10.1158/1535-7163.MCT-11-0675 Molecular Cancer Therapeutic Discovery Therapeutics Targeted Mutations in the ATR Pathway Define Agent- Specific Requirements for Cancer Cell Growth and Survival Deborah Wilsker1, Jon H. Chung1, Ivan Pradilla1, Eva Petermann2, Thomas Helleday2,3, and Fred Bunz1 Abstract Many anticancer agents induce DNA strand breaks or cause the accumulation of DNA replication intermediates. The protein encoded by ataxia-telangiectasia mutated and Rad 3-related (ATR) generates signals in response to these altered DNA structures and activates cellular survival responses. Accordingly, ATR has drawn increased attention as a potential target for novel therapeutic strategies designed to potentiate the effects of existing drugs. In this study, we use a unique panel of genetically modified human cancer cells to unambiguously test the roles of upstream and downstream components of the ATR pathway in the responses to common therapeutic agents. Upstream, the S-phase–specific cyclin-dependent kinase (Cdk) 2 was required for robust activation of ATR in response to diverse chemotherapeutic agents. While Cdk2-mediated ATR activation promoted cell survival after treatment with many drugs, signaling from ATR directly to the checkpoint kinase Chk1 was required for survival responses to only a subset of the drugs tested. These results show that specifically inhibiting the Cdk2/ATR/Chk1 pathway via distinct regulators can differentially sensitize cancer cells to a wide range of therapeutic agents. Mol Cancer Ther; 11(1); 98–107. Ó2011 AACR. Introduction stream pathways that control cell-cycle arrest and mediate cell survival (3). The phosphatidylinositol kinase–like kinase ATR is The central role played by ATR in the signaling path- directly activated by chemical agents that directly or ways turned on by therapeutic agents has been confirmed indirectly cause active DNA replication forks to stall by experimental systems in which ATR activity is limiting. -

Quantitative Modeling and Analysis of Drug Screening Data for Personalized Cancer Medicine
UNIVERSITY OF HELSINKI FACULTY OF MEDICINE QUANTITATIVE MODELING AND ANALYSIS OF DRUG SCREENING DATA FOR PERSONALIZED CANCER MEDICINE Lenalidomide Momelotinib Imiquimod Tacedinaline Tofacitinib Temsirolimus Ruxolitinib Roscovitine Tofacitinib Entinostat Gefitinib RoscovitineRuxolitinib Refametinib Pimasertib Sirolimus Selumetinib Tandutinib EverolimusNilotinib PF−04691502 NVP−BEZ235 Trametinib Palbociclib Melphalan Belinostat CUDC−101 AZD8055 Erlotinib Gefitinib Momelotinib Tacedinaline Cediranib SNS−032 Levamisole PF−04691502 Motesanib Panobinostat NVP−AUY922 Palbociclib OSI−027 Erlotinib OSI−027 Axitinib BIIB021 Imatinib Alvespimycin Masitinib TemsirolimusVorinostat Ponatinib Sunitinib Dasatinib Tanespimycin Melphalan Sorafenib Masitinib Pazopanib Sirolimus NVP−SNSAUY922−032 Vatalanib Dasatinib Regorafenib Everolimus Sunitinib MGCD−265 Panobinostat Tivozanib AZD8055 Foretinib NVP−BEZ235 Regorafenib Entinostat Prednisolone Tivozanib Dexamethasone Vincristine Cediranib Belinostat Methylprednisolone Pazopanib CUDC100−101 100 Vinblastine 100 100 Refametinib −40−20 0 20 40 Vatalanib Vorinostat Pimasertib MK1775 Sorafenib MGCD−80265 80 Trametinib ABT−751 80 80 Vandetanib Logistic Selumetinib Axitinib Alvespimycin DSS Triethylenemelamine S−trityl−L−cysteine 60 function3 60 DSS / sDSS 60 AA 60 Canertinib Tanespimycin Mechlorethamine Camptothecin Crizotinib Ponatinib Mitomycin C Paclitaxel Fingolimod 40 IC50 40 Lapatinib Calculation 40 40 Afatinib Imiquimod Patupilone AddictionDrug response score 100 Slope Chlorambucil DSS Tandutinib BIIB021 -

Antigen Binding Protein and Its Use As Addressing Product for the Treatment of Cancer
(19) TZZ 58Z9A_T (11) EP 2 589 609 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.: 08.05.2013 Bulletin 2013/19 C07K 16/28 (2006.01) (21) Application number: 11306416.6 (22) Date of filing: 03.11.2011 (84) Designated Contracting States: (72) Inventors: AL AT BE BG CH CY CZ DE DK EE ES FI FR GB •Beau-Larvor, Charlotte GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO 74520 Jonzier Epagny (FR) PL PT RO RS SE SI SK SM TR • Goetsch, Liliane Designated Extension States: 74130 Ayze (FR) BA ME (74) Representative: Regimbeau (83) Declaration under Rule 32(1) EPC (expert 20, rue de Chazelles solution) 75847 Paris Cedex 17 (FR) (71) Applicant: PIERRE FABRE MEDICAMENT 92100 Boulogne-Billancourt (FR) (54) Antigen binding protein and its use as addressing product for the treatment of cancer (57) The present invention relates to an antigen bind- of Axl, being internalized into the cell. The invention also ing protein, in particular a monoclonal antibody, capable comprises the use of said antigen binding protein as an of binding specifically to the protein Axl as well as the addressing product in conjugation with other anti- cancer amino and nucleic acid sequences coding for said pro- compounds,such as toxins, radio- elements ordrugs, and tein. From one aspect, the invention relates to an antigen the use of same for the treatment of certain cancers. binding protein, or antigen binding fragments, capable of binding specifically to Axl and, by inducing internalization EP 2 589 609 A1 Printed by Jouve, 75001 PARIS (FR) EP 2 589 609 A1 Description [0001] The present invention relates to a novel antigen binding protein, in particular a monoclonal antibody, capable of binding specifically to the protein Axl as well as the amino and nucleic acid sequences coding for said protein. -

Some Chemicals That Cause Tumours of the Urinary Tract in Rodents Some Chemicals That Cause Tumours of the Urinary Tract in Rodents Volume 119
SOME CHEMICALS THAT CAUSE TUMOURS SOME CHEMICALS THAT OF THE URINARY TRACT IN RODENTS TRACT IN RODENTS OF THE URINARY SOME CHEMICALS THAT CAUSE TUMOURS OF THE URINARY TRACT IN RODENTS VOLUME 119 IARC MONOGRAPHS ON THE EVALUATION OF CARCINOGENIC RISKS TO HUMANS SOME CHEMICALS THAT CAUSE TUMOURS OF THE URINARY TRACT IN RODENTS VOLUME 119 This publication represents the views and expert opinions of an IARC Working Group on the Evaluation of Carcinogenic Risks to Humans, which met in Lyon, 6–13 June 2017 LYON, FRANCE - 2019 IARC MONOGRAPHS ON THE EVALUATION OF CARCINOGENIC RISKS TO HUMANS IARC MONOGRAPHS In 1969, the International Agency for Research on Cancer (IARC) initiated a programme on the evaluation of the carcinogenic risk of chemicals to humans involving the production of critically evaluated monographs on individual chemicals. The programme was subsequently expanded to include evaluations of carcinogenic risks associated with exposures to complex mixtures, lifestyle factors and biological and physical agents, as well as those in specific occupations. The objective of the programme is to elaborate and publish in the form of monographs critical reviews of data on carcinogenicity for agents to which humans are known to be exposed and on specific exposure situations; to evaluate these data in terms of human risk with the help of international working groups of experts in carcinogenesis and related fields; and to indicate where additional research efforts are needed. The lists of IARC evaluations are regularly updated and are available on the Internet athttp:// monographs.iarc.fr/. This programme has been supported since 1982 by Cooperative Agreement U01 CA33193 with the United States National Cancer Institute, Department of Health and Human Services. -

United States Patent (10) Patent No.: US 9,605,003 B2 Castro Et Al
USOO9605 003B2 (12) United States Patent (10) Patent No.: US 9,605,003 B2 Castro et al. (45) Date of Patent: Mar. 28, 2017 (54) HETEROCYCLIC COMPOUNDS AND USES 5,294,612 A 3, 1994 Bacon et al. THEREOF 5,310,731 A 5/1994 Olsson et al. 5,364,862 A 11/1994 Spada et al. 5,420,419 A 5, 1995 Wood (71) Applicant: Infinity Pharmaceuticals, Inc., 5,428,125 A 6/1995 Hefner, Jr. et al. Cambridge, MA (US) 5,442,039 A 8/1995 Hefner, Jr. et al. 5,504,103 A 4/1996 Bonjouklian et al. (72) Inventors: Alfredo C. Castro, Woburn, MA (US); 5,506,347 A 4/1996 Erion et al. Catherine A. Evans, Somerville, MA 3: A 1999. St. al (US); Andre Lescarbeau, Somerville, 5.593.997 A 1/1997 f MA (US); Tao Liu, Wellesley, MA 5,646,128. A 7/1997 Firestein et al. (US); Daniel A. Snyder, Somerville, 5,652,366 A 7/1997 Spada et al. MA (US); Martin R. Tremblay, 39 A s 3. E. et al Melrose, MA (US); Pingda) Ren, San 5,674,998. A 10/1997 Boyeragwat et al.et al. Diego, CA (US); Yi Liu, San Diego, 5,686,455. A 1 1/1997 Adams et al. CA (US); Liansheng Li, San Diego, 5,736,554. A 4/1998 Spada et al. CA (US); Katrina Chan, Fremont, CA 5,747,235 A 5/1998 Farid et al. (US) 5,756,711 A 5/1998 Zilch et al. 5,763,596 A 6/1998 Boyer et al. -

(12) United States Patent �(1O) Patent No.: �US 8,173,115 B2 Decuzzi Et Al
(12) United States Patent (1o) Patent No.: US 8,173,115 B2 Decuzzi et al. (45) Date of Patent: *May 8, 2012 (54) PARTICLE COMPOSITIONS WITH A OTHER PUBLICATIONS PRE-SELECTED CELL INTERNALIZATION International Search Report and Written Opinion mailed Jun. 3, MODE 2009, in PCT/US2008/071470, 15 pages. (75) Inventors: Paolo Decuzzi, Bari (IT); Mauro U.S. Appl. No. 12/110,515, filed Apr. 28, 2008, Ferrari et al. Ferrari, Houston, TX (US) Champion et al., "Making polymeric micro- and nanoparticles of complex shapes," PHAS, Jul. 17, 2007, 104(29):11901-11904. (73) Assignee: The Board of Regents of The Champion et al., "Role of target geometry in phagocytosis," PHAS, University of Texas System, Austin, TX Mar. 28, 2006, 103(13):4930-4934. Cohen et al., "Microfabrication of Silicon-Based Nanoporous Par- (US) ticulates for Medical Applications," Biomedical Microdevices, 2003, 5(3):253-259. (*) Notice: Subject to any disclaimer, the term of this Conner et al., "Regulated portals of entry into the cell," Nature, Mar. patent is extended or adjusted under 35 6, 2003, 422:37-44. U.S.C. 154(b) by 390 days. Decuzzi et al,. "The role of specific and non-specific interactions in This patent is subject to a terminal dis- receptor-mediated endocytosis of nanoparticles," Biomaterials, claimer. 2007, 28:2915-2922. Decuzzi et al., "A Theoretical Model for the Margination of Particles (21) Appl. No.: 12/181,759 within Blood Vessels," Annals of Biomedical Engineering, Feb. 2005, 33(2):179-190. (22) Filed: Jul. 29, 2008 Decuzzi et al., "Adhesion of Microfabricated Particles of Vascular Endothelium: A Parametric Analysis," Annals of Biomedical Engi- (65) Prior Publication Data neering, Jun. -

( 12 ) United States Patent
US009889209B2 (12 ) United States Patent ( 10 ) Patent No. : US 9 ,889 ,209 B2 Mirkin et al. (45 ) Date of Patent: Feb . 13 , 2018 ( 54 ) NANOCONJUGATES ABLE TO CROSS THE 4 ,469 , 863 A 9 / 1984 Ts ' o et al . BLOOD - BRAIN BARRIER 4 ,476 , 301 A 10 / 1984 Imbach et al. 4 ,489 ,055 A 12 / 1984 Couvreur et al. 4 ,496 ,689 A 1 / 1985 Mitra ( 75 ) Inventors : Chad A . Mirkin , Wilmette , IL (US ) ; 4 ,587 ,044 A 5 / 1986 Miller et al . Caroline H . Ko, Chicago , IL (US ) ; 4 ,605 ,735 A 8 / 1986 Miyoshi et al . Alexander Stegh , Chicago , IL (US ) ; 4 ,640 , 835 A 2 / 1987 Shimizu et al. 4 ,667 , 025 A 5 / 1987 Miyoshi et al . David A . Giljohann , Chicago , IL (US ); 4 ,670 ,417 A 6 / 1987 Iwasaki et al. Janina Luciano , Champaign , IL (US ) ; 4 ,762 , 779 A 8 / 1988 Snitman Samuel A . Jensen , Bloomington , MN 4 ,789 , 737 A 12 / 1988 Miyoshi et al . (US ) 4 ,791 , 192 A 12 / 1988 Nakagawa et al. 4 , 824 , 941 A 4 / 1989 Gordon et al. (73 ) Assignee: NORTHWESTERN UNIVERSITY , 4 , 828 , 979 A 5 / 1989 Kievan et al. 4 , 835 , 263 A 5 / 1989 Nguyen et al . Evanston , IL (US ) 4 , 845 , 205 A 7 / 1989 Huynh Dinh et al . 4 ,876 , 335 A 10 / 1989 Yamane et al . ( * ) Notice: Subject to any disclaimer, the term of this 4 , 904 , 582 A 2 / 1990 Tullis patent is extended or adjusted under 35 4 , 948 , 882 A 8 / 1990 Ruth U . S . C . 154 ( b ) by 0 days . -

Applications of Microarrays with Toxicologically Relevant Genes (Tox
Mutation Research 549 (2004) 101–113 Applications of microarrays with toxicologically relevant genes (tox genes) for the evaluation of chemical toxicants in Sprague Dawley rats in vivo and human hepatocytes in vitro Larry D. Kier, Robin Neft, Lei Tang, Robert Suizu, Tari Cook, Kathy Onsurez, Karen Tiegler, Yumiko Sakai, Michael Ortiz, Tim Nolan, Usha Sankar, Albert P. Li∗ PHASE-1 Molecular Toxicology, Inc., Santa Fe, NM 87505, USA Received 3 September 2003; received in revised form 9 November 2003; accepted 10 November 2003 Abstract Microarrays with toxicologically relevant genes (tox genes) have been developed in our laboratory for toxicogenomics studies in rat, dog and man. The genes were chosen using published information as well as a discovery process for genes responsive to toxic treatments using transcription profiling experiments conducted with rats and dogs. In addition to published information human tox genes were derived from rat tox genes based on gene homology. Using the microarray with rat-specific tox genes, a database containing gene expression, histopathology, and clinical chemistry findings has been generated for 89 compounds. Analysis of the database indicates that treatment with toxic compounds induces specific gene expression patterns. Dose- and time-dependent response relationships in gene expression were observed for treatment with toxic compounds. Gene expression at 24 h was found to correlate well with organ toxicity observed at 72 h. Mining of the database led to the selection of specific groups of genes (predictive gene sets) whose expression patterns are predictive of organ toxicity with a high degree of accuracy (approximately 90%). The data also provide insight on toxic mechanism and gene regulation pathways.