The Taxonomic Report
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Rare Native Animals of RI
RARE NATIVE ANIMALS OF RHODE ISLAND Revised: March, 2006 ABOUT THIS LIST The list is divided by vertebrates and invertebrates and is arranged taxonomically according to the recognized authority cited before each group. Appropriate synonomy is included where names have changed since publication of the cited authority. The Natural Heritage Program's Rare Native Plants of Rhode Island includes an estimate of the number of "extant populations" for each listed plant species, a figure which has been helpful in assessing the health of each species. Because animals are mobile, some exhibiting annual long-distance migrations, it is not possible to derive a population index that can be applied to all animal groups. The status assigned to each species (see definitions below) provides some indication of its range, relative abundance, and vulnerability to decline. More specific and pertinent data is available from the Natural Heritage Program, the Rhode Island Endangered Species Program, and the Rhode Island Natural History Survey. STATUS. The status of each species is designated by letter codes as defined: (FE) Federally Endangered (7 species currently listed) (FT) Federally Threatened (2 species currently listed) (SE) State Endangered Native species in imminent danger of extirpation from Rhode Island. These taxa may meet one or more of the following criteria: 1. Formerly considered by the U.S. Fish and Wildlife Service for Federal listing as endangered or threatened. 2. Known from an estimated 1-2 total populations in the state. 3. Apparently globally rare or threatened; estimated at 100 or fewer populations range-wide. Animals listed as State Endangered are protected under the provisions of the Rhode Island State Endangered Species Act, Title 20 of the General Laws of the State of Rhode Island. -

Insect Survey of Four Longleaf Pine Preserves
A SURVEY OF THE MOTHS, BUTTERFLIES, AND GRASSHOPPERS OF FOUR NATURE CONSERVANCY PRESERVES IN SOUTHEASTERN NORTH CAROLINA Stephen P. Hall and Dale F. Schweitzer November 15, 1993 ABSTRACT Moths, butterflies, and grasshoppers were surveyed within four longleaf pine preserves owned by the North Carolina Nature Conservancy during the growing season of 1991 and 1992. Over 7,000 specimens (either collected or seen in the field) were identified, representing 512 different species and 28 families. Forty-one of these we consider to be distinctive of the two fire- maintained communities principally under investigation, the longleaf pine savannas and flatwoods. An additional 14 species we consider distinctive of the pocosins that occur in close association with the savannas and flatwoods. Twenty nine species appear to be rare enough to be included on the list of elements monitored by the North Carolina Natural Heritage Program (eight others in this category have been reported from one of these sites, the Green Swamp, but were not observed in this study). Two of the moths collected, Spartiniphaga carterae and Agrotis buchholzi, are currently candidates for federal listing as Threatened or Endangered species. Another species, Hemipachnobia s. subporphyrea, appears to be endemic to North Carolina and should also be considered for federal candidate status. With few exceptions, even the species that seem to be most closely associated with savannas and flatwoods show few direct defenses against fire, the primary force responsible for maintaining these communities. Instead, the majority of these insects probably survive within this region due to their ability to rapidly re-colonize recently burned areas from small, well-dispersed refugia. -

ANOTHER NEW EUPHYES from the SOUTHERN UNITED STATES COASTAL PLAIN (HESPERIIDAE) the Southern Atlantic and Gulf Coastal Plains Ar
Journal of the Lepidopteri'ts' Society .50(1 ), 1996, 46- 53 ANOTHER NEW EUPHYES FROM THE SOUTHERN UNITED STATES COASTAL PLAIN (HESPERIIDAE) JOHN A. SHUEY The Nature ConselVancy, Indiana Field Office, 1330 West 38th Street, Indianapolis, Indiana 46208, USA ABSTRACT. The taxon Euphyes dukesi calhouni Shuey, new subspecies endemic to FIOlida, is described. This subspecies is amply differentiated from Euphyes dukesi dukesi and the two taxa are allopatric. In northeaste rn F lorida and sout eastern G eorgia, whe re their known ranges closely approach one another, there is almost no evidence of inte r gradation. Euphyes dukesi calhouni is limited to swamp habitats that support large stands of the sedge hostplants, various Rhynchospora and Carex specie:; (Cype raceae). Additional key words: biogeography, we tlands, conservation. The southern Atlantic and Gulf Coastal Plains are rich regions for wetland butterflies, especially for genera such as Euphyes, Poanes, and Problema. For example , as currently known, eight named Euphyes spe cies or subspecies occur in the wetlands of these coastal plains. Four of these taxa are restricted to the coastal plain: Euphyes palatka palatka (Edwards), Euphyes palatka klotsi Mille r, Harvey and Miller, Euphyes berryi (Bell), and Euphyes bayensis Shuey. Just as interesting as their limited coastal distributions is the presence in these wetland skippers of well differentiated p eripheral populations, many of which have only recently bee n recognized and described. These peripheral populations are most probably the end result of allopatric diffe rentiation. For example, Euphyes palatka klotsi repre sents its spe cies on a few of the lower Florida Keys, separated from the nominate mainland subspecies by just tens of miles. -

Purple Lovegrass (Eragrostis Spectabilis)
Purple lovegrass ¤ The common name and Latin name are relatable. Eragrostis is derived from “Eros”, Eragrostis spectabilis the Greek word for love, and “Agrostis”, Family: Poaceae Genus: Eragrostis Species: spectabilis the Greek word for grass. Average Height: 24 inches Bloom Time: July and August Elevation Range: All elevations of the Piedmont, less common at high elevations. Geologic/Soil Associations: Generalist. Does well in nutrient-poor, sandy, rocky, or gravelly soil. Soil Drainage Regime: Xeric, dry-mesic, and mesic, well drained. Aspect: Full sun. East, South, & West. Rarely on fully exposed north facing xeric slopes. Habitat Associations: River shores and bars, riverside prairies, prairies in powerline right-of-ways, dry woodlands and barrens, clearings, fields, roadsides, hot and dry landscape restorations in urban spaces and natural area preserves, and other open, disturbed habitats. Common in the Piedmont. ¤ 6 or more florets per spikelet (best observed with hand lens) Flora Associations: This tough little bunch-grass grows in the harshest of roadside conditions, even where winter road salt is applied. It can also thrive alongside black walnut trees where many plants cannot. It is joined in these rough environs by its fellow stalwarts; little bluestem (Schizachyrium scoparium), Virginia wild strawberry (Fragaria virginiana), St. John’s-wort (Hypericum spp.), winged sumac (Rhus copallinum) and common yarrow (Achillea borealis). In less toxic spaces, such as powerline right-of -ways, purple lovegrass associates closely with many more species, including butterfly-weed (Asclepias tuberosa), and pasture thistle (Cirsium pumilum). Purple lovegrass is dependent on the nutrient-poor, dry conditions it favors. On moist fertile ground taller species would soon shade it out. -

Phylogeny and Biogeography of Euphyes Scudder (Hesperiidae)
JOURNAL OF THE LEPIDOPTERISTS' SOCIETY Volume 47 1993 Number 4 Journal of the Lepidopterists Society 47(4), 1993, 261-278 PHYLOGENY AND BIOGEOGRAPHY OF EUPHYES SCUDDER (HESPERIIDAE) JOHN A. SHUEyl Great Lakes Environmental Center, 739 Hastings, Traverse City, Michigan 49684 ABSTRACT. The 20 species of Euphyes were analyzed phylogenetically and were found to fall into four monophyletic species groups, each of which is defined by one or more apomorphic characters. The peneia group contains Euphyes peneia (Godman), E. eberti Mielke, E. leptosema (Mabille), E. fumata Mielke, E. singularis (Herrich-Schiiffer), and E. cornelius (Latreille). The subferruginea group contains E. subferruginea Mielke, E. antra Evans, and E. cherra Evans. The dion group contains E. dion (Edwards), E. dukesi (Lindsey), E. bayensis Shuey, E. pilatka (Edwards), E. berryi (Bell), and E. con spicua (Edwards). The vestris group contains E. vestris (Boisduval), E. chamuli Freeman, E. bimacula (Grote and Robinson), and E arpa (Boisduval and Leconte). Euphyes ampa Evans could not be placed confidently w .nin this framework. Geographic distribution of each speci~s group suggests that exchange between South America and North America took place at least twice. The two Caribbean Basin species (E. singularis, E. cornelius) share a common ancestor with E. peneia, a species found in Central and South America. This suggests a vicariant event involving Central America and the Greater Antilles. The dion and vestris groups show strong patterns of alJopatric differentiation, suggesting that the isolation and subsequent differentiation of peripheral populations has played an important role in the development of the extant species. Additional key words: evolution, cladistics, wetlands, vicariance biogeography, pop ulation differentiation. -

Host Plants of Poanes Melane (Hesperiidae)
386 JOURNAL OF THE LEPIDOPTERISTS' SOCIETY SMEDLEY AND THOMAS EISNER, Section of Neurobiology and Behavior, Cornell Uni versity, Ithaca, New York 14853, USA. Received for publication 28 December 1993; revised and accepted 10 April 1994. Journal of the Lepidopterists' Society 48(4), 1994,386-388 HOST PLANTS OF POANES MELANE (HESPERIIDAE) Additional key words: grasses, Poaceae, skipper, biology. Poanes melane (Edwards), the umber skipper [formerly Para try tone melane (Burns 1992)], is found along the Coastal Range of California and in the foothills of the Sierra Nevada. In rural areas adults are found in grassy habitats along streams and in forests (Emmel & Emmel 1973). This species is common in urban and suburban areas, most likely because its host plants (grasses; Poaceae) are abundant and well-maintained in lawns (Heppner 1972, Brown 1984). This source of host plants could become especially important during the summer generation, a dry season in California when many grasses are no longer green. Poanes melane larvae are known to feed on several Cs and C, grasses, including Cynodon dactylon (L.) Pers. (C,), Deschampsia caespitosa (L.) Beauv. (Ca), Lamarckia aurea L. (Moench) (Ca), and Stenotaphrum secundatum Kuntze (C,), and one sedge (Cyperaceae), Carex spissa Bailey (Brown 1984, Scott 1986). Adult female P. melane lay eggs singly on the undersurfaces of grass blades. The mature larva is approximately 30 mm long with a brown head and dusky yellow-green or tan body mottled with black punctations of varying sizes (Comstock & Dammers 1931, Emmel & Emmel 1973). Larvae have a blackish mid-dorsal line, a cream lateral stripe, and three dark lines on each side (Scott 1976). -
Butterflies of North Carolina - Twenty-Eighth Approximation 159
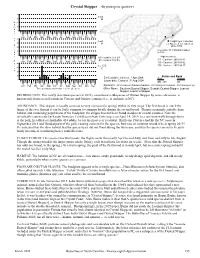
Crystal Skipper Atrytonopsis quinteri 40 n=0 30 M N 20 u m 10 b e 0 r 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 Jan Feb Mar Apr May Jun Jul Aug Sep Oct Nov Dec • o 40 • f n=0 = Sighting or Collection 30 P x• = Not seen nor collected F since 1980 l 20 i 5 records / 32 individuals added g 10 to 28th h 0 t 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 NC counties: 2 or 2% High counts of: 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 SC counties: 0 or 0% 414 - Carteret - 2019-04-14 D Jan Feb Mar Apr May Jun Jul Aug Sep Oct Nov Dec a 40 100 - Carteret - 2001-05-02 t n=123 100 - Carteret - 2003-04-17 e 30 C s 20 10 Status and Rank Earliest date: Carteret 1 Apr 2008 State Global 0 Latest date: Carteret 31 Aug 2004 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 SR - S1 G1 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 Jan Feb Mar Apr May Jun Jul Aug Sep Oct Nov Dec Synonym: Atrytonopsis hianna loammi, Atrytonopsis loammi, Atrytonopsis sp. -

Conservation of the Arogos Skipper, Atrytone Arogos Arogos (Lepidoptera: Hesperiidae) in Florida Marc C
Conservation of the Arogos Skipper, Atrytone arogos arogos (Lepidoptera: Hesperiidae) in Florida Marc C. Minno St. Johns River Water Management District P.O. Box 1429, Palatka, FL 32177 [email protected] Maria Minno Eco-Cognizant, Inc., 600 NW 35th Terrace, Gainesville, FL 32607 [email protected] ABSTRACT The Arogos skipper is a rare and declining butterfly found in native grassland habitats in the eastern and mid- western United States. Five distinct populations of the butterfly occur in specific parts of the range. Atrytone arogos arogos once occurred from southern South Carolina through eastern Georgia and peninsular Florida as far south as Miami. This butterfly is currently thought to be extirpated from South Carolina and Georgia. The six known sites in Florida for A. arogos arogos are public lands with dry prairie or longleaf pine savanna having an abundance of the larval host grass, Sorghastrum secundum. Colonies of the butterfly are threat- ened by catastrophic events such as wild fires, land management activities or no management, and the loss of genetic integrity. The dry prairie preserves of central Florida will be especially important to the recovery of the butterfly, since these are some of the largest and last remaining grasslands in the state. It may be possible to create new colonies of the Arogos skipper by releasing wild-caught females or captive-bred individuals into currently unoccupied areas of high quality habitat. INTRODUCTION tered colonies were found in New Jersey, North Carolina, South Carolina, Florida, and Mississippi. The three re- gions where the butterfly was most abundant included The Arogos skipper (Atrytone arogos) is a very locally the New Jersey pine barrens, peninsular Florida, and distributed butterfly that occurs only in the eastern and southeastern Mississippi. -

Download the Poweshiek Skipperling Status Assessment Update
STATUS ASSESSMENT UPDATE (2010) Poweshiek Skipperling Oarisma poweshiek (Parker) (Lepidoptera: Hesperiidae) Illinois, Indiana, Iowa, Michigan, Minnesota North Dakota, South Dakota, Wisconsin Contract #301818M448 By Gerald Selby Ecological and GIS Services 807 North W Street Indianola, IA 50125 For U.S. Fish and Wildlife Service Twin Cities Ecological Services Field Office 4101 E. 80th St. Bloomington, MN 55425 November 2010 Disclaimer This document is a compilation of biological data and a description of past, present, and likely future threats to Poweshiek skipperling (Oarisma poweshiek). It does not represent a decision by the U.S. Fish and Wildlife Service (Service) on whether this taxon should be designated as a candidate species for listing as threatened or endangered under the Federal Endangered Species Act. That decision will be made by the Service after reviewing this document; other relevant biological and threat data not included herein; and all relevant laws, regulations, and policies. The result of the decision will be posted on the Service's Region 3 Web site (refer to: http://www.fws.gov/midwest/endangered/lists/concern.html). If designated as a candidate species, the taxon will subsequently be added to the Service's candidate species list that is periodically published in the Federal Register and posted on the World Wide Web (refer to: http://endangered.fws.gov/wildlife.html). Even if the taxon does not warrant candidate status it should benefit from the conservation recommendations that are contained in this document. Suggested Citation: Selby, G. 2010. Status assessment update (2010): Poweshiek skipperling (Oarisma poweshiek (Parker)) (Lepidoptera: Hesperiidae). Prepared for Twin Cities Ecological Services Field Office, U.S. -

How to Use This Checklist
How To Use This Checklist Swallowtails: Family Papilionidae Special Note: Spring and Summer Azures have recently The information presented in this checklist reflects our __ Pipevine Swallowtail Battus philenor R; May - Sep. been recognized as separate species. Azure taxonomy has not current understanding of the butterflies found within __ Zebra Swallowtail Eurytides marcellus R; May - Aug. been completely sorted out by the experts. Cleveland Metroparks. (This list includes all species that have __ Black Swallowtail Papilio polyxenes C; May - Sep. __ Appalachian Azure Celastrina neglecta-major h; mid - late been recorded in Cuyahoga County, and a few additional __ Giant Swallowtail Papilio cresphontes h; rare in Cleveland May; not recorded in Cuy. Co. species that may occur here.) Record you observations and area; July - Aug. Brush-footed Butterflies: Family Nymphalidae contact a naturalist if you find something that may be of __ Eastern Tiger Swallowtail Papilio glaucus C; May - Oct.; __ American Snout Libytheana carinenta R; June - Oct. interest. females occur as yellow or dark morphs __ Variegated Fritillary Euptoieta claudia R; June - Oct. __ Spicebush Swallowtail Papilio troilus C; May - Oct. __ Great Spangled Fritillary Speyeria cybele C; May - Oct. Species are listed taxonomically, with a common name, a Whites and Sulphurs: Family Pieridae __ Aphrodite Fritillary Speyeria aphrodite O; June - Sep. scientific name, a note about its relative abundance and flight __ Checkered White Pontia protodice h; rare in Cleveland area; __ Regal Fritillary Speyeria idalia X; no recent Ohio records; period. Check off species that you identify within Cleveland May - Oct. formerly in Cleveland Metroparks Metroparks. __ West Virginia White Pieris virginiensis O; late Apr. -

Native Grasses Benefit Butterflies and Moths Diane M
AFNR HORTICULTURAL SCIENCE Native Grasses Benefit Butterflies and Moths Diane M. Narem and Mary H. Meyer more than three plant families (Bernays & NATIVE GRASSES AND LEPIDOPTERA Graham 1988). Native grasses are low maintenance, drought Studies in agricultural and urban landscapes tolerant plants that provide benefits to the have shown that patches with greater landscape, including minimizing soil erosion richness of native species had higher and increasing organic matter. Native grasses richness and abundance of butterflies (Ries also provide food and shelter for numerous et al. 2001; Collinge et al. 2003) and butterfly species of butterfly and moth larvae. These and moth larvae (Burghardt et al. 2008). caterpillars use the grasses in a variety of ways. Some species feed on them by boring into the stem, mining the inside of a leaf, or IMPORTANCE OF LEPIDOPTERA building a shelter using grass leaves and silk. Lepidoptera are an important part of the ecosystem: They are an important food source for rodents, bats, birds (particularly young birds), spiders and other insects They are pollinators of wild ecosystems. Terms: Lepidoptera - Order of insects that includes moths and butterflies Dakota skipper shelter in prairie dropseed plant literature review – a scholarly paper that IMPORTANT OF NATIVE PLANTS summarizes the current knowledge of a particular topic. Native plant species support more native graminoid – herbaceous plant with a grass-like Lepidoptera species as host and food plants morphology, includes grasses, sedges, and rushes than exotic plant species. This is partially due to the host-specificity of many species richness - the number of different species Lepidoptera that have evolved to feed on represented in an ecological community, certain species, genus, or families of plants. -

100 Characters
40 Review and Update of Non-mollusk Invertebrate Species in Greatest Need of Conservation: Final Report Leon C. Hinz Jr. and James N. Zahniser Illinois Natural History Survey Prairie Research Institute University of Illinois 30 April 2015 INHS Technical Report 2015 (31) Prepared for: Illinois Department of Natural Resources State Wildlife Grant Program (Project Number T-88-R-001) Unrestricted: for immediate online release. Prairie Research Institute, University of Illinois at Urbana Champaign Brian D. Anderson, Interim Executive Director Illinois Natural History Survey Geoffrey A. Levin, Acting Director 1816 South Oak Street Champaign, IL 61820 217-333-6830 Final Report Project Title: Review and Update of Non-mollusk Invertebrate Species in Greatest Need of Conservation. Project Number: T-88-R-001 Contractor information: University of Illinois at Urbana/Champaign Institute of Natural Resource Sustainability Illinois Natural History Survey 1816 South Oak Street Champaign, IL 61820 Project Period: 1 October 2013—31 September 2014 Principle Investigator: Leon C. Hinz Jr., Ph.D. Stream Ecologist Illinois Natural History Survey One Natural Resources Way, Springfield, IL 62702-1271 217-785-8297 [email protected] Prepared by: Leon C. Hinz Jr. & James N. Zahniser Goals/ Objectives: (1) Review all SGNC listing criteria for currently listed non-mollusk invertebrate species using criteria in Illinois Wildlife Action Plan, (2) Assess current status of species populations, (3) Review criteria for additional species for potential listing as SGNC, (4) Assess stressors to species previously reviewed, (5) Complete draft updates and revisions of IWAP Appendix I and Appendix II for non-mollusk invertebrates. T-88 Final Report Project Title: Review and Update of Non-mollusk Invertebrate Species in Greatest Need of Conservation.