Lysosomal SLC46A3 Modulates Hepatic Cytosolic Copper Homeostasis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Rise and Fall of the Bovine Corpus Luteum
University of Nebraska Medical Center DigitalCommons@UNMC Theses & Dissertations Graduate Studies Spring 5-6-2017 The Rise and Fall of the Bovine Corpus Luteum Heather Talbott University of Nebraska Medical Center Follow this and additional works at: https://digitalcommons.unmc.edu/etd Part of the Biochemistry Commons, Molecular Biology Commons, and the Obstetrics and Gynecology Commons Recommended Citation Talbott, Heather, "The Rise and Fall of the Bovine Corpus Luteum" (2017). Theses & Dissertations. 207. https://digitalcommons.unmc.edu/etd/207 This Dissertation is brought to you for free and open access by the Graduate Studies at DigitalCommons@UNMC. It has been accepted for inclusion in Theses & Dissertations by an authorized administrator of DigitalCommons@UNMC. For more information, please contact [email protected]. THE RISE AND FALL OF THE BOVINE CORPUS LUTEUM by Heather Talbott A DISSERTATION Presented to the Faculty of the University of Nebraska Graduate College in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy Biochemistry and Molecular Biology Graduate Program Under the Supervision of Professor John S. Davis University of Nebraska Medical Center Omaha, Nebraska May, 2017 Supervisory Committee: Carol A. Casey, Ph.D. Andrea S. Cupp, Ph.D. Parmender P. Mehta, Ph.D. Justin L. Mott, Ph.D. i ACKNOWLEDGEMENTS This dissertation was supported by the Agriculture and Food Research Initiative from the USDA National Institute of Food and Agriculture (NIFA) Pre-doctoral award; University of Nebraska Medical Center Graduate Student Assistantship; University of Nebraska Medical Center Exceptional Incoming Graduate Student Award; the VA Nebraska-Western Iowa Health Care System Department of Veterans Affairs; and The Olson Center for Women’s Health, Department of Obstetrics and Gynecology, Nebraska Medical Center. -

A Computational Approach for Defining a Signature of Β-Cell Golgi Stress in Diabetes Mellitus
Page 1 of 781 Diabetes A Computational Approach for Defining a Signature of β-Cell Golgi Stress in Diabetes Mellitus Robert N. Bone1,6,7, Olufunmilola Oyebamiji2, Sayali Talware2, Sharmila Selvaraj2, Preethi Krishnan3,6, Farooq Syed1,6,7, Huanmei Wu2, Carmella Evans-Molina 1,3,4,5,6,7,8* Departments of 1Pediatrics, 3Medicine, 4Anatomy, Cell Biology & Physiology, 5Biochemistry & Molecular Biology, the 6Center for Diabetes & Metabolic Diseases, and the 7Herman B. Wells Center for Pediatric Research, Indiana University School of Medicine, Indianapolis, IN 46202; 2Department of BioHealth Informatics, Indiana University-Purdue University Indianapolis, Indianapolis, IN, 46202; 8Roudebush VA Medical Center, Indianapolis, IN 46202. *Corresponding Author(s): Carmella Evans-Molina, MD, PhD ([email protected]) Indiana University School of Medicine, 635 Barnhill Drive, MS 2031A, Indianapolis, IN 46202, Telephone: (317) 274-4145, Fax (317) 274-4107 Running Title: Golgi Stress Response in Diabetes Word Count: 4358 Number of Figures: 6 Keywords: Golgi apparatus stress, Islets, β cell, Type 1 diabetes, Type 2 diabetes 1 Diabetes Publish Ahead of Print, published online August 20, 2020 Diabetes Page 2 of 781 ABSTRACT The Golgi apparatus (GA) is an important site of insulin processing and granule maturation, but whether GA organelle dysfunction and GA stress are present in the diabetic β-cell has not been tested. We utilized an informatics-based approach to develop a transcriptional signature of β-cell GA stress using existing RNA sequencing and microarray datasets generated using human islets from donors with diabetes and islets where type 1(T1D) and type 2 diabetes (T2D) had been modeled ex vivo. To narrow our results to GA-specific genes, we applied a filter set of 1,030 genes accepted as GA associated. -

Transcriptional Control of Tissue-Resident Memory T Cell Generation
Transcriptional control of tissue-resident memory T cell generation Filip Cvetkovski Submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the Graduate School of Arts and Sciences COLUMBIA UNIVERSITY 2019 © 2019 Filip Cvetkovski All rights reserved ABSTRACT Transcriptional control of tissue-resident memory T cell generation Filip Cvetkovski Tissue-resident memory T cells (TRM) are a non-circulating subset of memory that are maintained at sites of pathogen entry and mediate optimal protection against reinfection. Lung TRM can be generated in response to respiratory infection or vaccination, however, the molecular pathways involved in CD4+TRM establishment have not been defined. Here, we performed transcriptional profiling of influenza-specific lung CD4+TRM following influenza infection to identify pathways implicated in CD4+TRM generation and homeostasis. Lung CD4+TRM displayed a unique transcriptional profile distinct from spleen memory, including up-regulation of a gene network induced by the transcription factor IRF4, a known regulator of effector T cell differentiation. In addition, the gene expression profile of lung CD4+TRM was enriched in gene sets previously described in tissue-resident regulatory T cells. Up-regulation of immunomodulatory molecules such as CTLA-4, PD-1, and ICOS, suggested a potential regulatory role for CD4+TRM in tissues. Using loss-of-function genetic experiments in mice, we demonstrate that IRF4 is required for the generation of lung-localized pathogen-specific effector CD4+T cells during acute influenza infection. Influenza-specific IRF4−/− T cells failed to fully express CD44, and maintained high levels of CD62L compared to wild type, suggesting a defect in complete differentiation into lung-tropic effector T cells. -

Downloaded from USCS Tables (
bioRxiv preprint doi: https://doi.org/10.1101/318329; this version posted February 4, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. The selection arena in early human blastocysts resolves the pluripotent inner cell mass Manvendra Singh1, Thomas J. Widmann2, Vikas Bansal7, Jose L. Cortes2, Gerald G. Schumann3, Stephanie Wunderlich4, Ulrich Martin4, Marta Garcia-Canadas2, Jose L. Garcia- Perez2,5*, Laurence D. Hurst6*#, Zsuzsanna Izsvák1* 1 Max-Delbrück-Center for Molecular Medicine in the Helmholtz Society, Robert-Rössle- Strasse 10, 13125 Berlin, Germany. 2 GENYO. Centre for Genomics and Oncological Research: Pfizer/University of Granada/Andalusian Regional Government, PTS Granada, 18016 Granada, Spain. 3 Paul-Ehrlich-Institute, Division of Medical Biotechnology, Paul-Ehrlich-Strasse 51-59, 63225 Langen, Germany. 4Center for Regenerative Medicine Hannover Medical School (MHH) Carl-Neuberg-Str.1, Building J11, D-30625 Hannover, Germany 5Institute of Genetics and Molecular Medicine (IGMM), University of Edinburgh, Crewe Road, Edinburgh EH4 2XU, United Kingdom 6 The Milner Centre for Evolution, Department of Biology and Biochemistry, University of Bath, Bath, Somerset, UK, BA2 7AY. 7 Institute of Medical Systems Biology, Center for Molecular Neurobiology (ZMNH), University Medical Center Hamburg-Eppendorf (UKE), 20246, Hamburg, Germany. *Corresponding authors *Zsuzsanna Izsvák Max Delbrück Center for Molecular Medicine Robert Rössle Strasse 10, 13092 Berlin, Germany Telefon: +49 030-9406-3510 Fax: +49 030-9406-2547 email: [email protected] http://www.mdcberlin.de/en/research/research_teams/mobile_dna/index.html and *Laurence D. -

SLC46A3 As a Potential Predictive Biomarker for Antibody–Drug
Published OnlineFirst August 21, 2018; DOI: 10.1158/1078-0432.CCR-18-1300 Cancer Therapy: Preclinical Clinical Cancer Research SLC46A3 as a Potential Predictive Biomarker for Antibody–Drug Conjugates Bearing Noncleavable Linked Maytansinoid and Pyrrolobenzodiazepine Warheads Krista Kinneer1, John Meekin1, Arnaud C. Tiberghien2, Yu-Tzu Tai3, Sandrina Phipps4, Christine Mione Kiefer4, Marlon C. Rebelatto5, Nazzareno Dimasi4, Alyssa Moriarty6, Kyriakos P. Papadopoulos6, Sriram Sridhar5, Stephen J. Gregson2, Michael J. Wick6, Luke Masterson2, Kenneth C. Anderson3, Ronald Herbst1, Philip W. Howard2, and David A. Tice1 Abstract Purpose: Antibody–drug conjugates (ADC) utilizing non- also examined in patient-derived xenograft and in vitro cleavable linker drugs have been approved for clinical use, and models of acquired T-DM1 resistance and multiple myeloma several are in development targeting solid and hematologic bone marrow samples by RT-PCR. malignancies including multiple myeloma. Currently, there Results: Loss of SLC46A3 expressionwasfoundtobea are no reliable biomarkers of activity for these ADCs other than mechanismofinnateandacquiredresistancetoADCs presence of the targeted antigen. We observed that certain cell bearing DM1 and SG3376. Sensitivity was restored in refrac- lines are innately resistant to such ADCs, and sought to tory lines upon introduction of SLC46A3, suggesting that uncover the underlying mechanism of resistance. expression of SLC46A3 maybemorepredictiveofactivity Experimental Design: The expression of 43 lysosomal than target antigen levels alone. Interrogation of primary membrane target genes was evaluated in cell lines resistant multiple myeloma samples indicated a range of SLC46A3 to ADCs bearing the noncleavable linker, pyrrolobenzodiaze- expression, including samples with undetectable levels like pine payload SG3376, in vitro. The functional relevance of multiple myeloma cell lines resistant to BCMA-targeting SLC46A3, a lysosomal transporter of noncleavable ADC DM1andSG3376ADCs. -

Supplementary Table S4. FGA Co-Expressed Gene List in LUAD
Supplementary Table S4. FGA co-expressed gene list in LUAD tumors Symbol R Locus Description FGG 0.919 4q28 fibrinogen gamma chain FGL1 0.635 8p22 fibrinogen-like 1 SLC7A2 0.536 8p22 solute carrier family 7 (cationic amino acid transporter, y+ system), member 2 DUSP4 0.521 8p12-p11 dual specificity phosphatase 4 HAL 0.51 12q22-q24.1histidine ammonia-lyase PDE4D 0.499 5q12 phosphodiesterase 4D, cAMP-specific FURIN 0.497 15q26.1 furin (paired basic amino acid cleaving enzyme) CPS1 0.49 2q35 carbamoyl-phosphate synthase 1, mitochondrial TESC 0.478 12q24.22 tescalcin INHA 0.465 2q35 inhibin, alpha S100P 0.461 4p16 S100 calcium binding protein P VPS37A 0.447 8p22 vacuolar protein sorting 37 homolog A (S. cerevisiae) SLC16A14 0.447 2q36.3 solute carrier family 16, member 14 PPARGC1A 0.443 4p15.1 peroxisome proliferator-activated receptor gamma, coactivator 1 alpha SIK1 0.435 21q22.3 salt-inducible kinase 1 IRS2 0.434 13q34 insulin receptor substrate 2 RND1 0.433 12q12 Rho family GTPase 1 HGD 0.433 3q13.33 homogentisate 1,2-dioxygenase PTP4A1 0.432 6q12 protein tyrosine phosphatase type IVA, member 1 C8orf4 0.428 8p11.2 chromosome 8 open reading frame 4 DDC 0.427 7p12.2 dopa decarboxylase (aromatic L-amino acid decarboxylase) TACC2 0.427 10q26 transforming, acidic coiled-coil containing protein 2 MUC13 0.422 3q21.2 mucin 13, cell surface associated C5 0.412 9q33-q34 complement component 5 NR4A2 0.412 2q22-q23 nuclear receptor subfamily 4, group A, member 2 EYS 0.411 6q12 eyes shut homolog (Drosophila) GPX2 0.406 14q24.1 glutathione peroxidase -

Epistasis-Driven Identification of SLC25A51 As a Regulator of Human
ARTICLE https://doi.org/10.1038/s41467-020-19871-x OPEN Epistasis-driven identification of SLC25A51 as a regulator of human mitochondrial NAD import Enrico Girardi 1, Gennaro Agrimi 2, Ulrich Goldmann 1, Giuseppe Fiume1, Sabrina Lindinger1, Vitaly Sedlyarov1, Ismet Srndic1, Bettina Gürtl1, Benedikt Agerer 1, Felix Kartnig1, Pasquale Scarcia 2, Maria Antonietta Di Noia2, Eva Liñeiro1, Manuele Rebsamen1, Tabea Wiedmer 1, Andreas Bergthaler1, ✉ Luigi Palmieri2,3 & Giulio Superti-Furga 1,4 1234567890():,; About a thousand genes in the human genome encode for membrane transporters. Among these, several solute carrier proteins (SLCs), representing the largest group of transporters, are still orphan and lack functional characterization. We reasoned that assessing genetic interactions among SLCs may be an efficient way to obtain functional information allowing their deorphanization. Here we describe a network of strong genetic interactions indicating a contribution to mitochondrial respiration and redox metabolism for SLC25A51/MCART1, an uncharacterized member of the SLC25 family of transporters. Through a combination of metabolomics, genomics and genetics approaches, we demonstrate a role for SLC25A51 as enabler of mitochondrial import of NAD, showcasing the potential of genetic interaction- driven functional gene deorphanization. 1 CeMM Research Center for Molecular Medicine of the Austrian Academy of Sciences, Vienna, Austria. 2 Laboratory of Biochemistry and Molecular Biology, Department of Biosciences, Biotechnologies and Biopharmaceutics, -

Molecular Bases of Drug Resistance in Hepatocellular Carcinoma
cancers Review Molecular Bases of Drug Resistance in Hepatocellular Carcinoma Jose J.G. Marin 1,2,* , Rocio I.R. Macias 1,2 , Maria J. Monte 1,2 , Marta R. Romero 1,2, Maitane Asensio 1, Anabel Sanchez-Martin 1, Candela Cives-Losada 1, Alvaro G. Temprano 1, Ricardo Espinosa-Escudero 1, Maria Reviejo 1, Laura H. Bohorquez 1 and Oscar Briz 1,2,* 1 Experimental Hepatology and Drug Targeting (HEVEFARM) Group, University of Salamanca, IBSAL, 37007 Salamanca, Spain; [email protected] (R.I.R.M.); [email protected] (M.J.M.); [email protected] (M.R.R.); [email protected] (M.A.); [email protected] (A.S.-M.); [email protected] (C.C.-L.); [email protected] (A.G.T.); [email protected] (R.E.-E.); [email protected] (M.R.); [email protected] (L.H.B.) 2 Center for the Study of Liver and Gastrointestinal Diseases (CIBERehd), Carlos III National Institute of Health, 28029 Madrid, Spain * Correspondence: [email protected] (J.J.G.M.); [email protected] (O.B.); Tel.: +34-663182872 (J.J.G.M.); +34-923294674 (O.B.) Received: 4 June 2020; Accepted: 20 June 2020; Published: 23 June 2020 Abstract: The poor outcome of patients with non-surgically removable advanced hepatocellular carcinoma (HCC), the most frequent type of primary liver cancer, is mainly due to the high refractoriness of this aggressive tumor to classical chemotherapy. Novel pharmacological approaches based on the use of inhibitors of tyrosine kinases (TKIs), mainly sorafenib and regorafenib, have provided only a modest prolongation of the overall survival in these HCC patients. -
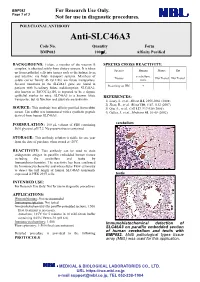
Anti-SLC46A3 Code No
BMP082 For Research Use Only. Page 1 of 3 Not for use in diagnostic procedures. POLYCLONAL ANTIBODY Anti-SLC46A3 Code No. Quantity Form BMP082 100 L Affinity Purified BACKGROUND: Folate, a member of the vitamin B SPECIES CROSS REACTIVITY: complex, is obtained solely from dietary sources. It is taken Species Human Mouse Rat up from epithelial cells into tissues such as the kidney, liver, and intestine via folate transport systems. Members of cerebellum, Tissues Not Tested Not Tested solute carrier family 46 (SLC46) are folate transporters. testis Several mutations in the SLC46A1 gene are found in Reactivity on IHC + patients with hereditary folate malabsorption. SLC46A2, also known as TSCOT/Ly110, is reported to be a thymic epithelial marker in mice. SLC46A3 is a known folate REFERENCES: transporter, but its function and substrate are unknown. 1) Lasry, I., et al., Blood 112, 2055-2061 (2008) 2) Zhao, R., et al., Blood 110, 1147-1152 (2007) SOURCE: This antibody was affinity purified from rabbit 3) Qiu, A., et al., Cell 127, 917-928 (2006) serum. The rabbit was immunized with a synthetic peptide 4) Geller, J., et al., Medicine 81, 51-68 (2002) derived from human SLC46A3. FORMULATION: 100 L volume of PBS containing cerebellum 50% glycerol, pH 7.2. No preservative is contained. STORAGE: This antibody solution is stable for one year from the date of purchase when stored at -20oC. REACTIVITY: This antibody can be used to stain endogenous antigen in paraffin embedded human tissues including the cerebellum and testis by Immunohistochemistry. The reactivity has been confirmed by Immunocytochemistry and intracellular Flow cytometry to detect the full length of human SLC46A3 transiently expressed in HEK 293T cells. -

Development and Characterization of the SLC46A3 Knockout Mouse Line
Development and Characterization of the SLC46A3 Knockout Mouse Line Summary (1024-character limit) The National Cancer Institute (NCI) seeks licensees for an SLC46A3 knockout mouse line. SLC46A3 is a solute carrier of the Major Facilitator Superfamily (MFS) and is thought to have roles in multiple diseases including nonalcoholic fatty liver disease, liver cancer and obesity. NIH Reference Number E-152-2021 Product Type Research Tools Keywords SLC46A3, Solute Carrier Family, Major Facilitator Superfamily, MFS, Nonalcoholic fatty liver disease, Obesity, Liver Cancer, Hepatocellular Carcinoma, Knockout Mouse, 2,3,7,8-tetracholodibenzo-p- dioxin, TCDD, Gonzalez Collaboration Opportunity This invention is available for licensing. Contact Michael Salgaller, Ph.D. NCI - National Cancer Institute 240-276-5476 [email protected] Description of Technology Nonalcoholic fatty liver disease is caused by several factors including 2,3,7,8-tetrachlorodibenzo-p- dioxin (TCDD), an environmental contaminant. TCDD causes lipid accumulation in humans by inducing the Solute Carrier Family 46 Member 3 (SLC46A3) gene expression. To effectively study TCDD- mediated lipid accumulation, research tools such as SLC46A3 knockout cells and animal models are required. Researchers at the National Cancer Institute (NCI) have developed an SLC46A3 knockout mouse line and demonstrated that TCDD-induced hepatic triglyceride accumulation was significantly reduced in the knockout mice compared to the wild type mice. This reduction in triglyceride accumulation was more pronounced when the mice were fed a high fat diet. The SLC46A3 knockout mouse line is therefore an effective tool to study TCDD-induced lipid accumulation, liver toxicity and nonalcoholic fatty liver NCI Technology Transfer Center https://techtransfer.cancer.gov/pdf/e-152-2021.pd f disease. -

William D. Hedrich Email: [email protected]
The Role of the Constitutive Androstane Receptor in Cyclophosphamide-based Treatment of Lymphomas Item Type dissertation Authors Hedrich, William Dominic Publication Date 2018 Abstract Cyclophosphamide (CPA) is an alkylating prodrug which has been utilized extensively in combination chemotherapies for the treatment of cancers and autoimmune disorders since its introduction to the market in the late 1950s. The metabolic conversion o... Keywords constitutive androstane receptor; CYP2B6; drug-drug interactions; Cyclophosphamide; Cytochrome P-450 CYP2B6; Drug Interactions Download date 07/10/2021 19:53:39 Link to Item http://hdl.handle.net/10713/8006 William D. Hedrich Email: [email protected] EDUCATION: Degree Institution Field of Study Date Bachelor of The Pennsylvania State Toxicology 2013 Science University Bachelor of The Pennsylvania State Biology-Vertebrate 2013 Science University Physiology Doctor of University of Maryland, Pharmaceutical 2018 Philosophy Baltimore Sciences ______________________________________________________________________________ RESEARCH EXPERIENCE Graduate Research Assistant Wang Lab, University of Maryland School of Pharmacy • The role of the constitutive androstane receptor in cyclophosphamide-based treatment of lymphomas • Inductive expression of drug metabolizing enzymes and transporters in human primary hepatocytes • Metabolism-mediated drug-drug interactions • In-vivo tumor xenograft models Hassan Lab, University of Maryland School of Pharmacy • Pharmacokinetics of l-THP as an experimental therapy -

Epigenetic Mechanisms Are Involved in the Oncogenic Properties of ZNF518B in Colorectal Cancer
Epigenetic mechanisms are involved in the oncogenic properties of ZNF518B in colorectal cancer Francisco Gimeno-Valiente, Ángela L. Riffo-Campos, Luis Torres, Noelia Tarazona, Valentina Gambardella, Andrés Cervantes, Gerardo López-Rodas, Luis Franco and Josefa Castillo SUPPLEMENTARY METHODS 1. Selection of genomic sequences for ChIP analysis To select the sequences for ChIP analysis in the five putative target genes, namely, PADI3, ZDHHC2, RGS4, EFNA5 and KAT2B, the genomic region corresponding to the gene was downloaded from Ensembl. Then, zoom was applied to see in detail the promoter, enhancers and regulatory sequences. The details for HCT116 cells were then recovered and the target sequences for factor binding examined. Obviously, there are not data for ZNF518B, but special attention was paid to the target sequences of other zinc-finger containing factors. Finally, the regions that may putatively bind ZNF518B were selected and primers defining amplicons spanning such sequences were searched out. Supplementary Figure S3 gives the location of the amplicons used in each gene. 2. Obtaining the raw data and generating the BAM files for in silico analysis of the effects of EHMT2 and EZH2 silencing The data of siEZH2 (SRR6384524), siG9a (SRR6384526) and siNon-target (SRR6384521) in HCT116 cell line, were downloaded from SRA (Bioproject PRJNA422822, https://www.ncbi. nlm.nih.gov/bioproject/), using SRA-tolkit (https://ncbi.github.io/sra-tools/). All data correspond to RNAseq single end. doBasics = TRUE doAll = FALSE $ fastq-dump -I --split-files SRR6384524 Data quality was checked using the software fastqc (https://www.bioinformatics.babraham. ac.uk /projects/fastqc/). The first low quality removing nucleotides were removed using FASTX- Toolkit (http://hannonlab.cshl.edu/fastxtoolkit/).