BISALAX (Bisacodyl)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Constipation: a Parent's Guide
CONSTIPATION: A PARENT’S GUIDE Twenty Questions About Constipation: Answers to Guide Parents and Professionals Constipation is the abnormally delayed or infrequent passage of hard stools. Most children, and many adults, too, become constipated from time to time. Often, the duration is short; occasionally it persists for months, even years. Although constipation can be uncomfortable, may create worry, and sometimes seem serious, fortunately it does not have long-term, troubling effects in most healthy children. This Booklet is designed to help you deal with childhood constipation by answering several questions and outlining management instructions for you to follow. Questions answered are: 1. What are the normal 11. What can we learn from patterns of bowel physical exam results? movements at 12. What is our treatment different ages? program for constipation? 2. What makes up bowel 13. How is the colon movements and how do cleaned out? they travel? 14. How are stools softened? 3. What is constipation? 15. Why is trying to have 4. When is constipation most bowel movements twice likely to occur? a day so important? 5. Why does constipation 16. What are the expected persist in some children? results of the treatment 6. Why would a child hold program? back stool and what 17. What do we do if the happens then? cleansing regimen is 7. How can proper toilet not successful? training help? 18. What is the long-term 8. Why would stool back up program for children in the colon? prone to constipation? 9. Why do some children 19. Does a special diet help have soiling accidents? resolve constipation? 10. -
Final Stakeholder List PDF 190 KB
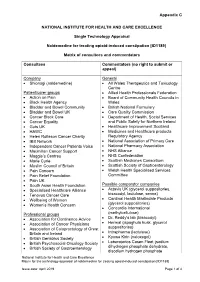
Appendix C NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Single Technology Appraisal Naldemedine for treating opioid-induced constipation [ID1189] Matrix of consultees and commentators Consultees Commentators (no right to submit or appeal) Company General • Shionogi (naldemedine) • All Wales Therapeutics and Toxicology Centre Patient/carer groups • Allied Health Professionals Federation • Action on Pain • Board of Community Health Councils in • Black Health Agency Wales • Bladder and Bowel Community • British National Formulary • Bladder and Bowel UK • Care Quality Commission • Cancer Black Care • Department of Health, Social Services • Cancer Equality and Public Safety for Northern Ireland • Guts UK • Healthcare Improvement Scotland • HAWC • Medicines and Healthcare products • Helen Rollason Cancer Charity Regulatory Agency • IBS Network • National Association of Primary Care • Independent Cancer Patients Voice • National Pharmacy Association • Macmillan Cancer Support • NHS Alliance • Maggie’s Centres • NHS Confederation • Marie Curie • Scottish Medicines Consortium • Muslim Council of Britain • Scottish Society of Gastroenterology • Pain Concern • Welsh Health Specialised Services • Pain Relief Foundation Committee • Pain UK • South Asian Health Foundation Possible comparator companies • Specialised Healthcare Alliance • Actavis UK (glycerol suppositories, • Tenovus Cancer Care bisacodyl, lactulose, senna) • Wellbeing of Women • Cardinal Health Martindale Products • Women’s Health Concern (glycerol suppositories) • Concordia International -

Ferring Pharmaceuticals Inc. Page 1 of 13 HIGHLIGHTS OF
HIGHLIGHTS OF PRESCRIBING INFORMATION ---------------------DOSAGE FORMS AND STRENGTHS---------------------- CLENPIQ oral solution: Each bottle contains 10 mg of sodium picosulfate, These highlights do not include all the information needed to use ® 3.5 g of magnesium oxide, and 12 g of anhydrous citric acid in 160 mL of CLENPIQ safely and effectively. See full prescribing information for solution (3) CLENPIQ. -------------------------------CONTRAINDICATIONS------------------------------ ® • Patients with severe reduced renal impairment (creatinine clearance less CLENPIQ (sodium picosulfate, magnesium oxide, and anhydrous citric than 30 mL/minute) (4, 5.3, 8.6) acid) oral solution • Gastrointestinal (GI) obstruction or ileus (4) Initial U.S. Approval: 2012 • Bowel perforation (4) • ----------------------------RECENT MAJOR CHANGES-------------------------- Toxic colitis or toxic megacolon (4) Indications and Usage (1) 08/2019 • Gastric retention (4) Dosage and Administration (2.1) 10/2019 • Hypersensitivity to any of the ingredients in CLENPIQ (4) Dosage and Administration (2.2) 08/2019, 10/2019 Dosage and Administration, Day-Before Dosage Regimen (2.3) -----------------------WARNINGS AND PRECAUTIONS------------------------ Removed 10/2019 • Risk of fluid and electrolyte abnormalities, arrhythmia, seizures, and renal Warnings and Precautions (5.1) 08/2019 impairment: Encourage adequate hydration, assess concurrent medications, and consider laboratory assessments prior to and after use. (5.1, 5.2, 5.3, 5.4, ----------------------------INDICATIONS AND USAGE--------------------------- 7.1) CLENPIQ® is a combination of sodium picosulfate, a stimulant laxative, and • Use in patients with renal impairment or taking concomitant medications magnesium oxide and anhydrous citric acid, which form magnesium citrate, that affect renal function: Use caution, ensure adequate hydration, and an osmotic laxative, indicated for cleansing of the colon as a preparation for consider testing. -

Laxatives for the Management of Constipation in People Receiving Palliative Care (Review)
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by UCL Discovery Laxatives for the management of constipation in people receiving palliative care (Review) Candy B, Jones L, Larkin PJ, Vickerstaff V, Tookman A, Stone P This is a reprint of a Cochrane review, prepared and maintained by The Cochrane Collaboration and published in The Cochrane Library 2015, Issue 5 http://www.thecochranelibrary.com Laxatives for the management of constipation in people receiving palliative care (Review) Copyright © 2015 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd. TABLE OF CONTENTS HEADER....................................... 1 ABSTRACT ...................................... 1 PLAINLANGUAGESUMMARY . 2 BACKGROUND .................................... 2 OBJECTIVES ..................................... 4 METHODS ...................................... 4 RESULTS....................................... 7 Figure1. ..................................... 8 Figure2. ..................................... 9 Figure3. ..................................... 10 DISCUSSION ..................................... 13 AUTHORS’CONCLUSIONS . 14 ACKNOWLEDGEMENTS . 14 REFERENCES ..................................... 15 CHARACTERISTICSOFSTUDIES . 17 DATAANDANALYSES. 26 ADDITIONALTABLES. 26 APPENDICES ..................................... 28 WHAT’SNEW..................................... 35 HISTORY....................................... 35 CONTRIBUTIONSOFAUTHORS . 36 DECLARATIONSOFINTEREST . 36 SOURCESOFSUPPORT . 36 DIFFERENCES -

Assessment Report on Ricinus Communis L., Oleum Final
2 February 2016 EMA/HMPC/572973/2014 Committee on Herbal Medicinal Products (HMPC) Assessment report on Ricinus communis L., oleum Final Based on Article 10a of Directive 2001/83/EC as amended (well-established use) Herbal substance(s) (binomial scientific name Ricinus communis L., oleum (castor oil) of the plant, including plant part) Herbal preparation Fatty oil obtained from seeds of Ricinus communis L. by cold expression Pharmaceutical forms Herbal preparation in liquid or solid dosage forms for oral use Rapporteur C. Purdel Peer-reviewer B. Kroes 30 Churchill Place ● Canary Wharf ● London E14 5EU ● United Kingdom Telephone +44 (0)20 3660 6000 Facsimile +44 (0)20 3660 5555 Send a question via our website www.ema.europa.eu/contact An agency of the European Union © European Medicines Agency, 2016. Reproduction is authorised provided the source is acknowledged. Table of contents Table of contents ................................................................................................................... 2 1. Introduction ....................................................................................................................... 4 1.1. Description of the herbal substance(s), herbal preparation(s) or combinations thereof .. 4 1.2. Search and assessment methodology ..................................................................... 6 2. Data on medicinal use ........................................................................................................ 6 2.1. Information about products on the market ............................................................. -

Chronic Constipation: an Evidence-Based Review
J Am Board Fam Med: first published as 10.3122/jabfm.2011.04.100272 on 7 July 2011. Downloaded from CLINICAL REVIEW Chronic Constipation: An Evidence-Based Review Lawrence Leung, MBBChir, FRACGP, FRCGP, Taylor Riutta, MD, Jyoti Kotecha, MPA, MRSC, and Walter Rosser MD, MRCGP, FCFP Background: Chronic constipation is a common condition seen in family practice among the elderly and women. There is no consensus regarding its exact definition, and it may be interpreted differently by physicians and patients. Physicians prescribe various treatments, and patients often adopt different over-the-counter remedies. Chronic constipation is either caused by slow colonic transit or pelvic floor dysfunction, and treatment differs accordingly. Methods: To update our knowledge of chronic constipation and its etiology and best-evidence treat- ment, information was synthesized from articles published in PubMed, EMBASE, and Cochrane Database of Systematic Reviews. Levels of evidence and recommendations were made according to the Strength of Recommendation taxonomy. Results: The standard advice of increasing dietary fibers, fluids, and exercise for relieving chronic constipation will only benefit patients with true deficiency. Biofeedback works best for constipation caused by pelvic floor dysfunction. Pharmacological agents increase bulk or water content in the bowel lumen or aim to stimulate bowel movements. Novel classes of compounds have emerged for treating chronic constipation, with promising clinical trial data. Finally, the link between senna abuse and colon cancer remains unsupported. Conclusions: Chronic constipation should be managed according to its etiology and guided by the best evidence-based treatment.(J Am Board Fam Med 2011;24:436–451.) copyright. Keywords: Chronic Constipation, Clinical Review, Evidence-Based Medicine, Family Medicine, Gastrointestinal Problems, Systematic Review The word “constipation” has varied meanings for was established in 1991 by Drossman et al, primar- different individuals. -

Immediate Hypersensitivity Reactions Caused by Drug Excipients: a Literature Review Caballero ML, Quirce S
REVIEWS Immediate Hypersensitivity Reactions Caused by Drug Excipients: A Literature Review Caballero ML, Quirce S Department of Allergy, La Paz University Hospital, IdiPAZ, Madrid, Spain J Investig Allergol Clin Immunol 2020; Vol. 30(2): 86-100 doi: 10.18176/jiaci.0476 Abstract The European Medicines Agency defines excipients as the constituents of a pharmaceutical form apart from the active substance. Immediate hypersensitivity reactions (IHRs) caused by excipients contained in the formulation of medications have been described. However, there are no data on the prevalence of IHRs due to drug excipients. Clinical manifestations of allergy to excipients can range from skin disorders to life-threatening systemic reactions. The aim of this study was to review the literature on allergy to pharmaceutical excipients and to record the IHRs described with various types of medications, specifically reactions due to the excipients contained in their formulations. The cases reported were sorted alphabetically by type of medication and excipient in order to obtain a list of the excipients most frequently involved for each type of medication. Key words: Allergy. Drug immediate hypersensitivity reaction. Excipient. Pharmaceutical excipients. Resumen La Agencia Europea de Medicamentos define los excipientes como los componentes de una forma farmacéutica diferenciados del principio activo. Se han descrito reacciones de hipersensibilidad inmediata causadas por los excipientes contenidos en la formulación de medicamentos. Sin embargo, no hay datos sobre la prevalencia de dichas reacciones. Las manifestaciones clínicas de la alergia a los excipientes pueden ir desde trastornos de la piel hasta reacciones sistémicas que ponen en peligro la vida. El objetivo de este estudio fue realizar una revisión de la literatura sobre la alergia a los excipientes farmacéuticos y recopilar las reacciones inmediatas descritas con diferentes tipos de medicamento, debido solo a excipientes contenidos en sus formulaciones. -

Prucalopride for the Treatment of Chronic Constipation in Women
Prucalopride for the Treatment of Chronic Constipation in Women: Single Technology Appraisal Response to consultation from Norgine Pharmaceuticals Ltd Comments on clinical assessment 1. The ICER for prucalopride of around £15,000 to £17,000 per QALY gained, whilst probably acceptable for an innovative medicine for a serious or life- threatening condition, is far in excess of what should be considered as acceptable for a laxative. As far as providing value for the NHS is concerned the ICER for Norgine‟s macrogol laxative Movicol is estimated at £250 per QALY gained1, which clearly provides much better value for money. Norgine would therefore question whether prucalopride should be recommended at all by NICE for use in the NHS in England and Wales. 2. If it is considered that prucalopride is cost-effective for use in the NHS in England and Wales, then the preliminary recommendations of the Advisory Committee are not sufficiently precise to avoid doubt as to what the guidance is intended to recommend. For example: (i) It is not clear what is meant by “a clinician with experience of treating chronic constipation.” Many clinicians treat chronic constipation and in numerical terms, nurses probably are involved to a greater extent in managing this condition in primary care than are doctors, and as a result probably have more experience in treating chronic constipation than do primary care physicians. Not all gastroenterologists in secondary care would necessarily qualify as experienced in treating chronic constipation, unless they specialise in the functional bowel disorders. Therefore we would suggest that initially at least the guidance should state that prucalopride therapy should only be initiated by a secondary care physician specialising in the treatment of the functional bowel disorders. -

ACR Manual on Contrast Media
ACR Manual On Contrast Media 2021 ACR Committee on Drugs and Contrast Media Preface 2 ACR Manual on Contrast Media 2021 ACR Committee on Drugs and Contrast Media © Copyright 2021 American College of Radiology ISBN: 978-1-55903-012-0 TABLE OF CONTENTS Topic Page 1. Preface 1 2. Version History 2 3. Introduction 4 4. Patient Selection and Preparation Strategies Before Contrast 5 Medium Administration 5. Fasting Prior to Intravascular Contrast Media Administration 14 6. Safe Injection of Contrast Media 15 7. Extravasation of Contrast Media 18 8. Allergic-Like And Physiologic Reactions to Intravascular 22 Iodinated Contrast Media 9. Contrast Media Warming 29 10. Contrast-Associated Acute Kidney Injury and Contrast 33 Induced Acute Kidney Injury in Adults 11. Metformin 45 12. Contrast Media in Children 48 13. Gastrointestinal (GI) Contrast Media in Adults: Indications and 57 Guidelines 14. ACR–ASNR Position Statement On the Use of Gadolinium 78 Contrast Agents 15. Adverse Reactions To Gadolinium-Based Contrast Media 79 16. Nephrogenic Systemic Fibrosis (NSF) 83 17. Ultrasound Contrast Media 92 18. Treatment of Contrast Reactions 95 19. Administration of Contrast Media to Pregnant or Potentially 97 Pregnant Patients 20. Administration of Contrast Media to Women Who are Breast- 101 Feeding Table 1 – Categories Of Acute Reactions 103 Table 2 – Treatment Of Acute Reactions To Contrast Media In 105 Children Table 3 – Management Of Acute Reactions To Contrast Media In 114 Adults Table 4 – Equipment For Contrast Reaction Kits In Radiology 122 Appendix A – Contrast Media Specifications 124 PREFACE This edition of the ACR Manual on Contrast Media replaces all earlier editions. -

Assessment Report on Cassia Senna L
European Medicines Agency Evaluation of Medicines for Human Use London, 27 April 2007 Doc. Ref. EMEA/HMPC/51868/2006 Corr. COMMITTEE ON HERBAL MEDICINAL PRODUCTS (HMPC) ASSESSMENT REPORT ON CASSIA SENNA L. AND CASSIA ANGUSTIFOLIA VAHL, FOLIUM Herbal substance Cassia senna L. (C. acutifolia Delile) [Alexandrian or Khartoum senna] or Cassia angustifolia Vahl [Tinnevelly senna], folium (senna leaf) or a mixture of the two species Herbal preparation dried leaflets, standardised; standardised herbal preparations thereof Pharmaceutical forms Herbal substance for oral preparation Rapporteur Dr C. Werner Assessor Dr B. Merz Superseded 7 Westferry Circus, Canary Wharf, London, E14 4HB, UK Tel. (44-20) 74 18 84 00 Fax (44-20) 75 23 70 51 E-mail: [email protected] http://www.emea.europa.eu ©EMEA 2007 Reproduction and/or distribution of this document is authorised for non commercial purposes only provided the EMEA is acknowledged TABLE OF CONTENTS I. Introduction 3 II. Clinical Pharmacology 3 II.1 Pharmacokinetics 3 II.1.1 Phytochemical characterisation 3 II.1.2 Absorption, metabolism and excretion 4 II.1.3 Progress of action 5 II.2 Pharmacodynamics 5 II.2.1 Mode of action 5 • Laxative effect 5 II.2.2 Interactions 7 III. Clinical Efficacy 7 III.1 Dosage 7 III.2 Clinical studies 8 III.2.1 Constipation 8 III.2.2 Irritable bowel syndrome 10 III.2.3 Bowel cleansing 11 III.3 Clinical studies in special populations 15 III.3.1 Use in children 15 III.3.2 Use during pregnancy and lactation 16 III.4 Traditional use 17 IV. -

Bowel Protocol for Hemodialysis
Clinical Dialysis/Patient Care/Bowel Protocol Hemodialysis Bowel Protocol for Hemodialysis Application: Constipation is a common problem for people on dialysis in part due to fluid restrictions and a low potassium diet which limits the types and amount of fiber in their diets. Medications such as phosphorus binders and opioids also contribute to constipation. Goal: The focus of the Bowel Protocol for Hemodialysis is to relieve and prevent constipation. Policy: 1. All patients on hemodialysis will be evaluated for constipation and given the appropriate recommendation. Bowel Protocol orders will be entered using ICD-10 K59.00. Procedure: 1. RD will interview patients on bowel movements as part of comprehensive assessments. 2. If a patient has mild constipation or Type 3 on the Bristol Stool Form Scale start option a, b or c one at a time, if effective, continue for maintenance: a. 1-3 tablespoons of cellulose fiber (Unifiber), ground flax or wheat bran daily. Start with 1 tablespoon per day, increasing by 1 tablespoon only as often as every 3 days. Do not exceed 3 tablespoons per day. b. 2 prunes per day. Limit to 2 per day to avoid high potassium. c. If patient has gastroparesis and/or fiber and prunes are not effective use docusate sodium (DOSS or Colace) 100mg/day increasing to up to 300mg/day. If no improvement move to number 3. Page 1 of 2 Northwest Kidney Centers Clinical Dialysis/Patient Care/Bowel Protocol Hemodialysis 3. If a patient has moderate constipation or Type 2 on the Bristol Stool Form Scale recommend: a. Polyethylene glycol (Miralax), 17gm powder in 4-8 ounces fluid daily. -

Drugs Used in Treating Constipation and IBS
Drugs used in treating constipation and IBS Define constipation Know the different symptoms of constipation Know the different lines of treatment of constipation Identify the different types of laxatives Discuss the pharmacokinetics, dynamics, side effects and uses of laxatives Discuss the difference between different treatment including bulk forming laxatives, osmotic laxatives, stimulant laxatives And stool softeners (lubricants). Define bowel syndrome (IBS). Identify the pharmacokinetics, dynamics, side effects and uses of drugs used for IBS. extra information and further explanation important doctors notes Drugs names Mnemonics Kindly check the editing file before studying this document constipation and IBS Very useful. Don’t miss it Infrequent defecation, often with straining and the passage of hard, uncomfortable stools. - May be accompanied by other symptoms: Abdominal discomfort and rectal pain, Flatulence, Loss of appetite, Lethargy & Depression. Decreased motility in colon: Decrease in water and fiber Difficulty in evacuation: Drug-induced: contents of diet. 1. Local painful conditions: 1. Anticholinergic agents “Unbalanced diet” anal fissures, piles. 2. Opioids 2. Lack of muscular 3. Iron exercise. 4. Antipsychotics. 5. Anti-depressant, anti- histamine, has anticholinergic effect Adequate fluid intake High fiber contents in diet Use drugs (laxatives or Regulation of bowel habit. purgatives more watery effect) Regular exercise Avoid drugs causing constipation. • Drugs that hasten the transit of food through the gastrointestinal tract are called (Or we say the drugs that increase GI motility) (محركات) or purgatives(ملينات) laxatives • Loosen stools and increase bowel movement Bulk forming laxatives (increase the size of stool ) • Increase volume of non-absorbable solid residue. Osmotic laxatives (cause water withdrawal by sugar or salt) • Increase water content in large intestine.