An Assessment of Marking Techniques for Odonates in the Family Calopterygidae
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Predictive Modelling of Spatial Biodiversity Data to Support Ecological Network Mapping: a Case Study in the Fens
Predictive modelling of spatial biodiversity data to support ecological network mapping: a case study in the Fens Christopher J Panter, Paul M Dolman, Hannah L Mossman Final Report: July 2013 Supported and steered by the Fens for the Future partnership and the Environment Agency www.fensforthefuture.org.uk Published by: School of Environmental Sciences, University of East Anglia, Norwich, NR4 7TJ, UK Suggested citation: Panter C.J., Dolman P.M., Mossman, H.L (2013) Predictive modelling of spatial biodiversity data to support ecological network mapping: a case study in the Fens. University of East Anglia, Norwich. ISBN: 978-0-9567812-3-9 © Copyright rests with the authors. Acknowledgements This project was supported and steered by the Fens for the Future partnership. Funding was provided by the Environment Agency (Dominic Coath). We thank all of the species recorders and natural historians, without whom this work would not be possible. Cover picture: Extract of a map showing the predicted distribution of biodiversity. Contents Executive summary .................................................................................................................... 4 Introduction ............................................................................................................................... 5 Methodology .......................................................................................................................... 6 Biological data ................................................................................................................... -

A Checklist of North American Odonata, 2021 1 Each Species Entry in the Checklist Is a Paragraph In- Table 2
A Checklist of North American Odonata Including English Name, Etymology, Type Locality, and Distribution Dennis R. Paulson and Sidney W. Dunkle 2021 Edition (updated 12 February 2021) A Checklist of North American Odonata Including English Name, Etymology, Type Locality, and Distribution 2021 Edition (updated 12 February 2021) Dennis R. Paulson1 and Sidney W. Dunkle2 Originally published as Occasional Paper No. 56, Slater Museum of Natural History, University of Puget Sound, June 1999; completely revised March 2009; updated February 2011, February 2012, October 2016, November 2018, and February 2021. Copyright © 2021 Dennis R. Paulson and Sidney W. Dunkle 2009, 2011, 2012, 2016, 2018, and 2021 editions published by Jim Johnson Cover photo: Male Calopteryx aequabilis, River Jewelwing, from Crab Creek, Grant County, Washington, 27 May 2020. Photo by Netta Smith. 1 1724 NE 98th Street, Seattle, WA 98115 2 8030 Lakeside Parkway, Apt. 8208, Tucson, AZ 85730 ABSTRACT The checklist includes all 471 species of North American Odonata (Canada and the continental United States) considered valid at this time. For each species the original citation, English name, type locality, etymology of both scientific and English names, and approximate distribution are given. Literature citations for original descriptions of all species are given in the appended list of references. INTRODUCTION We publish this as the most comprehensive checklist Table 1. The families of North American Odonata, of all of the North American Odonata. Muttkowski with number of species. (1910) and Needham and Heywood (1929) are long out of date. The Anisoptera and Zygoptera were cov- Family Genera Species ered by Needham, Westfall, and May (2014) and West- fall and May (2006), respectively. -

Identification Guide to the Australian Odonata Australian the to Guide Identification
Identification Guide to theAustralian Odonata www.environment.nsw.gov.au Identification Guide to the Australian Odonata Department of Environment, Climate Change and Water NSW Identification Guide to the Australian Odonata Department of Environment, Climate Change and Water NSW National Library of Australia Cataloguing-in-Publication data Theischinger, G. (Gunther), 1940– Identification Guide to the Australian Odonata 1. Odonata – Australia. 2. Odonata – Australia – Identification. I. Endersby I. (Ian), 1941- . II. Department of Environment and Climate Change NSW © 2009 Department of Environment, Climate Change and Water NSW Front cover: Petalura gigantea, male (photo R. Tuft) Prepared by: Gunther Theischinger, Waters and Catchments Science, Department of Environment, Climate Change and Water NSW and Ian Endersby, 56 Looker Road, Montmorency, Victoria 3094 Published by: Department of Environment, Climate Change and Water NSW 59–61 Goulburn Street Sydney PO Box A290 Sydney South 1232 Phone: (02) 9995 5000 (switchboard) Phone: 131555 (information & publication requests) Fax: (02) 9995 5999 Email: [email protected] Website: www.environment.nsw.gov.au The Department of Environment, Climate Change and Water NSW is pleased to allow this material to be reproduced in whole or in part, provided the meaning is unchanged and its source, publisher and authorship are acknowledged. ISBN 978 1 74232 475 3 DECCW 2009/730 December 2009 Printed using environmentally sustainable paper. Contents About this guide iv 1 Introduction 1 2 Systematics -

Ohio Damselfly Species Checklist
Ohio Damselfly Species Checklist Ohio has ~51 species of damselflies (Zygoptera). This is a statewide species checklist to encourage observations of damselflies for the Ohio Dragonfly Survey. Please submit photo observations to iNaturalist.org. More information can be found on our survey website at u.osu.edu/ohioodonatasurvey/ Broad Winged Damselflies (Calopterygidae) 1 Appalachian Jewelwing Calopteryx angustipennis 2 River Jewelwing Calopteryx aequabilis State Endangered 3 Ebony Jewelwing Calopteryx maculata 4 American Rubyspot Hetaerina americana 5 Smoky Rubyspot Hetaerina titia Pond Damselflies (Coenagrionidae) 6 Eastern Red Damsel Amphiagrion saucium 7 Blue-fronted Dancer Argia apicalis 8 Seepage Dancer Argia bipunctulata State Endangered 9 Powdered Dancer Argia moesta 10 Blue-ringed Dancer Argia sedula 11 Blue-tipped Dancer Argia tibialis 12 Dusky Dancer Argia translata 13 Violet Dancer Argia fumipennis violacea 14 Aurora Damsel Chromagrion conditum 15 Taiga Bluet Coenagrion resolutum 16 Turquoise Bluet Enallagma divagans 17 Hagen's Bluet Enallagma hageni 18 Boreal Bluet Enallagma boreale State Threatened 19 Northern Bluet Enallagma annexum State Threatened 20 Skimming Bluet Enallagma geminatum 21 Orange Bluet Enallagma signatum 22 Vesper Bluet Enallagma vesperum 23 Marsh Bluet Enallagma ebrium State Threatened 24 Stream Bluet Enallagma exsulans 25 Rainbow Bluet Enallagma antennatum 26 Tule Bluet Enallagma carunculatum 27 Atlantic Bluet Enallagma doubledayi 1 28 Familiar Bluet Enallagma civile 29 Double-striped Bluet Enallagma basidens -

From the Ebony Jewelwing Damsel
Comp. Parasitol. 71(2), 2004, pp. 141–153 Calyxocephalus karyopera g. nov., sp. nov. (Eugregarinorida: Actinocephalidae: Actinocephalinae) from the Ebony Jewelwing Damselfly Calopteryx maculata (Zygoptera: Calopterygidae) in Southeast Nebraska, U.S.A.: Implications for Mechanical Prey–Vector Stabilization of Exogenous Gregarine Development RICHARD E. CLOPTON Department of Natural Science, Peru State College, Peru, Nebraska 68421, U.S.A. (e-mail: [email protected]) ABSTRACT: Calyxocephalus karyopera g. nov., sp. nov. (Apicomplexa: Eugregarinorida: Actinocephalidae: Actino- cephalinae) is described from the Ebony Jewelwing Damselfly Calopteryx maculata (Odonata: Zygoptera: Calopteryigidae) collected along Turkey Creek in Johnson County, Nebraska, U.S.A. Calyxocephalus gen. n. is distinguished by the form of the epimerite complex: a terminal thick disk or linearly crateriform sucker with a distal apopetalus calyx of petaloid lobes and a short intercalating diamerite (less than half of the total holdfast length). The epimerite complex is conspicuous until association and syzygy. Association occurs immediately before syzygy and is cephalolateral and biassociative. Gametocysts are spherical with a conspicuous hyaline coat. Lacking conspicuous sporoducts they dehisce by simple rupture. Oocysts are axially symmetric, hexagonal dipyramidic in shape with slight polar truncations, bearing 6 equatorial spines, 1 at each equatorial vertex and 6 terminal spines obliquely inserted at each pole, 1 at each vertex created by polar truncation. The ecology of the C. karyopera–C. maculata host–parasite system provides a mechanism for mechanical prey–vector stabilization of exogenous gregarine development and isolation. KEY WORDS: Odonata, Zygoptera, Calopteryx maculata, damselfly, Apicomplexa, Eugregarinida, Actinocephalidae, Actinocephalinae, Calyxocephalus karyopera, new genus, new species, parasite evolution, biodiversity, species isolation, vector, transmission. -

The Classification and Diversity of Dragonflies and Damselflies (Odonata)*
Zootaxa 3703 (1): 036–045 ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Correspondence ZOOTAXA Copyright © 2013 Magnolia Press ISSN 1175-5334 (online edition) http://dx.doi.org/10.11646/zootaxa.3703.1.9 http://zoobank.org/urn:lsid:zoobank.org:pub:9F5D2E03-6ABE-4425-9713-99888C0C8690 The classification and diversity of dragonflies and damselflies (Odonata)* KLAAS-DOUWE B. DIJKSTRA1, GÜNTER BECHLY2, SETH M. BYBEE3, RORY A. DOW1, HENRI J. DUMONT4, GÜNTHER FLECK5, ROSSER W. GARRISON6, MATTI HÄMÄLÄINEN1, VINCENT J. KALKMAN1, HARUKI KARUBE7, MICHAEL L. MAY8, ALBERT G. ORR9, DENNIS R. PAULSON10, ANDREW C. REHN11, GÜNTHER THEISCHINGER12, JOHN W.H. TRUEMAN13, JAN VAN TOL1, NATALIA VON ELLENRIEDER6 & JESSICA WARE14 1Naturalis Biodiversity Centre, PO Box 9517, NL-2300 RA Leiden, The Netherlands. E-mail: [email protected]; [email protected]; [email protected]; [email protected]; [email protected] 2Staatliches Museum für Naturkunde Stuttgart, Rosenstein 1, 70191 Stuttgart, Germany. E-mail: [email protected] 3Department of Biology, Brigham Young University, 401 WIDB, Provo, UT. 84602 USA. E-mail: [email protected] 4Department of Biology, Ghent University, Ledeganckstraat 35, B-9000 Ghent, Belgium. E-mail: [email protected] 5France. E-mail: [email protected] 6Plant Pest Diagnostics Branch, California Department of Food & Agriculture, 3294 Meadowview Road, Sacramento, CA 95832- 1448, USA. E-mail: [email protected]; [email protected] 7Kanagawa Prefectural Museum of Natural History, 499 Iryuda, Odawara, Kanagawa, 250-0031 Japan. E-mail: [email protected] 8Department of Entomology, Rutgers University, Blake Hall, 93 Lipman Drive, New Brunswick, New Jersey 08901, USA. -
Dragonfly (Pg. 3-4) Head Eye Color
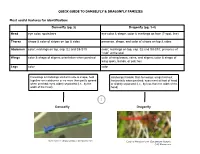
QUICK GUIDE TO DAMSELFLY & DRAGONFLY FAMILIES Most useful features for identification: Damselfly (pg. 2) Dragonfly (pg. 3-4) Head eye color; spots/bars eye color & shape; color & markings on face (T-spot, line) Thorax shape & color of stripes on top & sides presence, shape, and color of stripes on top & sides Abdomen color; markings on top, esp. S2 and S8-S10 color; markings on top, esp. S2 and S8-S10; presence of “club” at the end Wings color & shape of stigma; orientation when perched color of wing bases, veins, and stigma; color & shape of wing spots, bands, or patches Legs color color Forewings & hindwings similar in size & shape, held Hindwings broader than forewings; wings held out together over abdomen or no more than partly spread horizontally when perched; eyes meet at front of head when perched; eyes widely separated (i.e., by the or slightly separated (i.e., by less than the width of the width of the head) head) 1 Damselfly Dragonfly Vivid Dancer (Argia vivida); CAS Mazzacano Cardinal Meadowhawk (Sympetrum illotum); CAS Mazzacano DAMSELFLIES Wings narrow, stalked at base 2 Wings broad, colored, not stalked at base 3 Wings held askew Wings held together Broad-winged Damselfly when perched when perched (Calopterygidae); streams Spreadwing Pond Damsels (Coenagrionidae); (Lestidae); ponds ponds, streams Dancer (Argia); Wings held above abdomen; vivid colors streams 4 Wings held along Bluet (Enallagma); River Jewelwing (Calopteryx aequabilis); abdomen; mostly blue ponds CAS Mazzacano Dark abdomen with blue Forktail (Ischnura); tip; -

Calopteryx Splendens, C. Virgo) During Social Interaction: a Signal Or a Symptom?
Russian Entomol. J. 25(1): 103–120 © RUSSIAN ENTOMOLOGICAL JOURNAL, 2016 Behaviour of two demoiselle species (Calopteryx splendens, C. virgo) during social interaction: a signal or a symptom? Ïîâåäåíèå äâóõ âèäîâ ðàâíîêðûëûõ ñòðåêîç êðàñîòêè áëåñòÿùåé Calopteryx splendens Harris, 1780 è êðàñîòêè-äåâóøêè C. virgo Linnaeus, 1758 (Odonata, Calopterygidae) E.N. Panov1*, A.S. Opaev1, E.Yu. Pavlova1, V.A. Nepomnyashchikh2 Å.Í. Ïàíîâ1*, À.Ñ. Îïàåâ1, Å.Þ. Ïàâëîâà1, Â.À. Íåïîìíÿùèõ2 1 A.N. Severtsov Institute of Ecology and Evolution, Russian Academy of Sciences, Leninsky prosp., 33, Moscow, 119071 Russia. 1 Институт проблем экологии и эволюции им. А.Н. Северцова РАН, Ленинский просп., 33, Москва 119071 Россия. 2 Institute of Biology of Inland Waters, Russian Academy of Sciences, Borok, 152742 Russia. 2 Институт биологии внутренних вод РАН, п. Борок, Ярославская область, 152742 Россия. * Corresponding author. E-mail: [email protected] KEY WORDS: damselfly, agonistic behaviour, evolution of courtship behaviour, sexual selection. КЛЮЧЕВЫЕ СЛОВА: стрекоза, агонистическое поведение, эволюция поведения ухаживания, половой отбор. ABSTRACT. Based on the analysis of video record- ного характера. Подвержены серьезной критике ings, obtained during five field seasons, we suggest a трактовки функциональных объяснений поведения pattern of analytical description of damselfly (Zygoptera: стрекоз, господствующие в современной литерату- Calopterygidae) behaviour in a broad spectrum of so- ре по этим насекомым и основанные на откровен- cial contexts. The arrays of motor coordinations and ном антропоморфизме. Этому подходу противопо- their place in social interactions are studied compara- ставлен совершенно иной, призывающий к возвра- tively and in the temporal dynamic. The first description ту к оставленным в последние десятилетия методам of the full set of behaviours of Banded Demoiselles и принципам, разработанным ранее в рамках поле- Calopteryx splendens is given; the respective data for вой и аналитической этологии. -

Dragonflies & Damselflies
dragonflies & damselflies understanding an insect order by three essential facts Klaas-Douwe ‘KD’ B. Dijkstra Netherlands Centre for Biodiversity Naturalis enveloping eyes Anisoptera dragonflies different hindwing Zygoptera 2740 sp. Zygoptera opposed eyes damselflies fact one similar 5680 species in 2 suborders hindwing 20,000 Orthoptera; 160,000 Lepidoptera; 100,000s of Coleoptera & Hymenoptera evolution of Palaeoptera wingspan 15-70 cm Namurotypus sippeli Meganisoptera Protodonata small antennae node Ephemeroptera Aeshna cyanea unsegmented gripping cerci Calopterygidae Amphipterygidae advancement Lestidae Megapodagrionidae Coenagrionidae Bybee et al. (2008) 12S, 16S, COII (mitochondrion) 18S, 28S (nucleus) morphology branch thickness reflects species richness families Coenagrionidae Mecistogaster Anonisma Megaloprepus Libellulidae Coenagrionidae Coenagrionidae Erythromma near Lib. near Coen. Aeshnidae Gomphidae Odonata Platycnemididae Zygoptera Platycnemis dominated by Coenagrionoidea Calopterygidae Sapho Synlestidae Platycnemididae Chlorolestes Chlorocnemis Euphaeidae Euphaea Megapodagrionidae Philosina Bybee et al. (2008) Ware et al. (2007) Aeshnidae Macromiidae Corduliidae branch thickness reflects species richness families Libellulidae Libellulidae Coenagrionidae near Lib. near Coen. Libellulidae Aeshnidae Libellula Gomphidae Odonata Anisoptera Corduliidae Somatochlora dominated by Libelluloidea Libellulidae Coenagrionidae near Lib. near Coen. Gomphidae Aeshnidae Ophiogomphus Gomphidae Odonata Anisoptera Aeshnidae Aeshna “Aeshnoidea” -

Little Bluet
Species Status Assessment Class: Insecta Family: Coenagrionidae Scientific Name: Enallagma minusculum Common Name: Little bluet Species synopsis: The distribution of the little bluet (Enallagma minusculum) encompasses North Carolina, the northeastern United States, and southeastern Canada (Nikula et al. 2003). More specifically, the species is found in North Carolina, New York, Connecticut, Rhode Island, Massachusetts, New Hampshire, Maine, New Brunswick, Nova Scotia, and Prince Edward Island (NatureServe 2012, Abbott 2007). In New York, E. minusculum is now known to occur at three locations, two in Suffolk County and one in Queens County (New York Natural Heritage Program 2010). The species is known to inhabit ponds and lakes with sandy substrate, mainly coastal plain ponds with emergent vegetation along the shoreline (Carpenter 1991, Lam 2004). I. Status a. Current Legal Protected Status i. Federal ____Not Listed__ _________ Candidate: __No____ ii. New York ____Threatened; SGCN _ _______ b. Natural Heritage Program Rank i. Global ____G4__ _____ ii. New York ____S1__ ______ Tracked by NYNHP? __Yes___ Other Rank: IUCN Red List— Least Concern Status Discussion: White et al. (2010) suggests that the status remain S1 (5 or fewer occurrences, or few remaining acres or miles of stream, or factors demonstrably making it especially vulnerable to extinction rangewide or in New York State). 1 II. Abundance and Distribution Trends a. North America i. Abundance _____ declining _____increasing __ ___stable __X___ unknown ii. Distribution: _____ declining _____increasing __ ___stable __X___ unknown Time frame considered: _____Last assessment US 2006, Canada 2012__ ____ b. Regional i. Abundance _____ declining _____increasing __ ___stable ___X__ unknown ii. Distribution: _____ declining _____increasing __ X__ stable _____ unknown Regional Unit Considered:_____________Northeast________________________________ Time Frame Considered: ____Last assessment 2006____________________ c. -

Deciphering the Förster Dragonfly Collection Hadley Samarco, Erika Tucker P.H.D
Collection Detective: Deciphering the Förster Dragonfly Collection Hadley Samarco, Erika Tucker P.h.D. Fig. 1 Fig.2 Abstract Discussion Friedrich Förster was a german zoologist who traveled the globe In total, 180 specimens were identified from all around the around the turn of the twentieth century collecting hundreds of globe, spanning six different continents and 27 countries. dragonfly and damselfly specimens. After his death, his collection They belonged to six different species: Coenagrionidae, was donated to the University of Michigan for safe keeping, but Platycnemididae, Protoneuridae, Megapodagrionidae, has remained largely unexplored. In an effort to make the Euphaeidae, and Calopterygidae. Of all the dragonflies and collection and associated data accessible, we are digitizing the damselflies imaged, a bit less than half of them had available Forster collection, and using the data to map where he traveled. locations to be plotted on the map. Some of these locations To do this, we are systematically imaging the specimens alongside were vague, for example just identifying the country of their original labels and adding a scannable unique identification origin with no other details. The dragonflies and damselflies code to identify them within the university’s collection. These identified do not represent the entire Förster collection; for a images are then uploaded to a drive where the labels are complete exploration of the collection all specimens would transcribed and entered into an organized spreadsheet. The Results need to be analyzed in a similar manner. specimens are then returned to their collection case with their new Specimens with a known location were put on the map pictured in identification number. -

Ebony Jewelwing, Black-Winged Damselfly
EENY-693 Ebony Jewelwing, Black-Winged Damselfly (suggested common names) Calopteryx maculata (Beauvois, 1807) (Insecta: Odonata: Calopterygidae)1 Alfred Runkel, Nathan Burkett-Cadena, and Andrea Lucky2 Introduction Calopteryx maculata (Beauvois), the ebony jewelwing, is a large damselfly in the family Calopterygidae that is endemic to eastern North America. The ebony jewelwing has an iridescent green body with dark wings (Figures 1–3). Wings of the male ebony jewelwing are completely black, while wings of the female are smoky bronze with a distinct white spot (pterostigma) at the outer edge of the forewing (Figure 4). The combination of iridescent green body and dark wings distinguish this species from all other damselflies in the family Calopterygidae, and from other damselflies in North America. The ebony jewelwing is not listed as a species of concern. Figure 2. Male ebony jewelwing, Calopteryx maculata (Beauvois), resting on a leaf (lateral view). Credits: Nathan Burkett-Cadena, UF/IFAS Florida Medical Entomology Laboratory Synonymy Agrion maculatum (Beauvois 1805) Agrion virginica (Westwood 1837) Calopteryx virginica (Westwood 1837) Figure 1. Male ebony jewelwing, Calopteryx maculata (Beauvois), Calopteryx holosericea (Burmeister 1839) resting on a leaf (lateral view). Credits: Alfred Runkel, UF/IFAS Florida Medical Entomology Laboratory Calopteryx opaca (Say 1839) 1. This document is EENY-693, one of a series of the Department of Entomology and Nematology, UF/IFAS Extension. Original publication date November 2017. Visit the EDIS website at http://edis.ifas.ufl.edu. This document is also available on the Featured Creatures website at http:// entnemdept.ifas.ufl.edu/creatures/. 2. Alfred Runkel, field assistant, Department of Entomology and Nematology, Florida Medical Entomology Laboratory; Nathan Burkett-Cadena, Department of Entomology and Nematology, Florida Medical Entomology Laboratory; and Andrea Lucky, Department of Entomology and Nematology; UF/IFAS Extension, Gainesville, FL 32611.