EFTBA Veterinary Newsletter 37
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

PIAFFE in ENGLISH Totilas Or
PIAFFE IN ENGLISH magazine translation for the international reader 2010/1 magazine page 4 Totilas Or: Mr. Gal’s Big Appearance By Karin Schweiger took risks during his performance. Even though he could not prevent mistakes within Gold, silver, bronze – in 2009, the Dutch riders the flying changes at every stride, the entire decided the European championship dressage audience was enchanted by jet-black Totilas freestyle titles among themselves. Edward Gal and his prodigious movements. made history by achieving an all-time high of 90.70 points and opening the door to new His victory was a given and at no point in spheres of grading. The audience – and not danger. Totilas had earned it. 90.70 points! No just the Dutch spectators – was beyond itself one had believed this sound barrier could ever watching the battle between the three top be breached – it has been done, however. Gal contestants from the Netherlands. This was won gold which represents his first ever major the first time the stadium had ever been sold title. Dressage fans may decide for themselves out; tension could not have been built up in a if Erik Lette, president of the judges present, is more dramatic way: First, current winners of right by saying the following: “This was the the Classic Tour Adelinde Cornelissen and best competition we have ever seen. So many Parzival had to set the pace. Their great horses – and from various nations at performance included highstepping passages, that. It will prove beneficial for the future of a wonderfully impulsive extended trot and our sport.” All of the above is based on the rhythmic double pirouettes. -
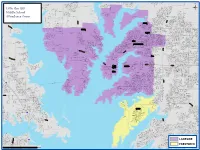
2019-2020 Middle School Zone
Z GAYLA CREEK DR HOLLIDAY LN E R BRA D FIN D CROSS OAK RANCH BLVD CH DR R R K P D H O E DR C N R TUMBLEWEED DR E SU D R N R C CALLIOPSIS ST A E ENCINO DR W R MILL CREEK RD Y CHISHOLM TRL GREGORY CREEK DR CYPRESS LHILLO DR FRISCO HILLS BLVD B B EPPRIGHT DR F B ST T I HAWKINS LN HAWKINS AUTRY DR A M E OAK SHORES DR LAKE HOLLOW DRLAKE GROVE DR L CORRAL ST WAGON TRL A LAKE MOSS LN R DRIFTERST S LITTLE RANCH RD CHEYENNEDR D LAKE FOREST TRL LLOYDS RD JILL CREEK DR SPRINGS DR T N W KING RANCH DR BENJAMIN CREEK DR LOGA N CACTUS TRL D I O LAKE POINT CT PAWNEEST OAK HILL CT V BIG HORN RD O CRYSTAL LAKE DR A WOUNDEDKNEE DR RICHMOND CIR L P SAGEBRUSH TRL LAKE WAY DRLAKE MEADOW LN EAGLEMONT DR K B K OAKSHORES CT S OAK VIEW DR K A PRAIRIE DR RIDGEDR H LITTLE ANNE DR GLORYVIEW RD E O YOSEMITE TRL PAINTST O KEYES LN AMETHYST DR E R V WATERS DR E C R E N RA T C L I NAYLORRD SIER RL O S L NE A A BRENDAN DR BRENDAN D M SP R M R Little Elm ISD R U TRAILBLAZER DR RED DR I HIGHLAND CIR O N O FOG G RIVERSIDE DR T L L TRANQUILITY CT CRYSTALCV E LLANO DR L MT EVANS DR D N A R Ü OAK SHORES CIR AMBER LN P BROOKE CT WHIRLWIND TRL S S SPRUCE ST U KOEHN DR R R L N CANYON LAKE DR D A D S P DYE RD FRISCO RANCH RD K T P E E L R BLUEWATERDR E A E A T O K CALMWOOD DR I CANYON DR R N 6 DR S R A G RIVER CR 24 R HICKMAN ST G DOE BRANCH RD LE L L T H P MICKEY LN L T 8 I A O H HIGH PLAINS DRL D C N O N V R S O 0 COSTA MESA DR N M Middle School P D MILL TOWNDR R R N YUK I I O T E 1 L A N N Z LONE PINE DR E F R LN NICHOLAS E A X L I O D Z E DR PEAK CIR MENOMINEE DR T R P G L -

T Am T Th T Be an C M in Fo Co Fa Gr W St Ch T Ra Sm in R No T Str W Fa
As a freelance writer, Alan Yuill Walker has spent The Scots & The Turf tells the story of the his life writing about racing and bloodstock. For amazing contribution made to the world of over forty years he was a regular contributor to Thoroughbred horseracing by the Scots and Horse & Hound and has had a long involvement those of Scottish ancestry, past and present. with the Thoroughbred Breeders’ Association. Throughout the years, this contribution has Other magazines/journals to which he has been across the board, from jockeys to trainers contributed on a regular basis include The and owners as well as some superb horses. British/European Racehorse, Stud & Stable, Currently, Scotland has a great ambassador in Pacemaker, The Thoroughbred Breeder and Mark Johnston, who has resurrected Middleham Thoroughbred Owner & Breeder. He was also in North Yorkshire as one of the country’s a leading contributor to The Bloodstock foremost training centres, while his jumping Breeders’ Annual Review. His previous books counterpart Alan King, the son of a Lanarkshire are Thoroughbred Studs of Great Britain, The farmer, is now based outside Marlborough. The History of Darley Stud Farms, Months of Misery greatest lady owner of jumpers in recent years Moments of Bliss, and Grey Magic. was Queen Elizabeth the Queen Mother, while Stirling-born Willie Carson was five-times champion jockey on the Flat. These are, of course, familiar names to any racing enthusiast but they represent just a small part of the Scottish connection that has influenced the Sport of Kings down the years. Recognition of the part played by those from north of the Border is long overdue and The Scots & The Turf now sets the record straight with a fascinating account of those who have helped make horseracing into the fabulous spectacle it is today. -

O Du Mein Österreich: Patriotic Music and Multinational Identity in The
O du mein Österreich: Patriotic Music and Multinational Identity in the Austro-Hungarian Empire by Jason Stephen Heilman Department of Music Duke University Date: _______________________ Approved: ______________________________ Bryan R. Gilliam, Supervisor ______________________________ Scott Lindroth ______________________________ James Rolleston ______________________________ Malachi Hacohen Dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the Department of Music in the Graduate School of Duke University 2009 ABSTRACT O du mein Österreich: Patriotic Music and Multinational Identity in the Austro-Hungarian Empire by Jason Stephen Heilman Department of Music Duke University Date: _______________________ Approved: ______________________________ Bryan R. Gilliam, Supervisor ______________________________ Scott Lindroth ______________________________ James Rolleston ______________________________ Malachi Hacohen An abstract of a dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the Department of Music in the Graduate School of Duke University 2009 Copyright by Jason Stephen Heilman 2009 Abstract As a multinational state with a population that spoke eleven different languages, the Austro-Hungarian Empire was considered an anachronism during the age of heightened nationalism leading up to the First World War. This situation has made the search for a single Austro-Hungarian identity so difficult that many historians have declared it impossible. Yet the Dual Monarchy possessed one potentially unifying cultural aspect that has long been critically neglected: the extensive repertoire of marches and patriotic music performed by the military bands of the Imperial and Royal Austro- Hungarian Army. This Militärmusik actively blended idioms representing the various nationalist musics from around the empire in an attempt to reflect and even celebrate its multinational makeup. -

Bally Cor (1965)
TesioPower jadehorse Bally Cor (1965) Vedette 19 GALOPIN Flying Duchess 3 ST SIMON King Tom 3 St Angela Adeline 11 Childwick (1890) Chattanooga 3 WELLINGTONIA Araucaria 3 Plaisanterie Trocadero 2 Poetess La Dorette 19 NEGOFOL (1906) Bertram 18 Robert The Devil Cast Off 1 Hoche HERMIT 5 Hermita Affection 19 Nebrouze (1899) Flageolet 6 Manoel Vestale 19 Nebuleuse Ventre St Gris 5 Navarre Noelie 17 Bois De Rose (1924) HERMIT 5 Friar's Balsam The Flower Of Dorset 2 Voter Barcaldine 23 Mavourneen Gaydene 1 Ballot (1904) Lowlander 19 Lowland Chief Bathilde 23 Cerito Doncaster 5 Merry Dance Highland Fling 14 Rose Leaves (1916) Toxophilite 3 Musket West Australian Mare 3 Trenton Goldsbrough 13 Frailty Flora McIvor 18 Colonial (1897) Sterling 12 Paradox Casuistry 1 Thankful Blossom HERMIT 5 The Apple Black Star 9 Cormac (1943) DOLLAR 1 Androcles Alabama 6 CAMBYSE Plutus 15 Cambuse Campeche 2 Gardefeu (1895) See Saw 6 Bruce Carine 3 Bougie The Heir Of Linne 21 La Lumiere Grande Mademoiselle 6 Chouberski (1902) LORD CLIFDEN 2 Petrarch Laura 10 The Bard Syrian 5 Magdalene My Mary 1 Campanule (1891) Beadsman 13 Rosicrucian Mme Eglantine 5 St Lucia Knowsley 3 Rose Of Tralee Vimiera 28 Sauge () GALOPIN 3 ST SIMON St Angela 11 St Damien HERMIT 5 DISTANT SHORE Land's End 9 Cheri (1898) Chattanooga 3 WELLINGTONIA Araucaria 3 Cromatella DOLLAR 1 Perla Pergola 8 Sainte Rose (1911) Androcles 6 CAMBYSE Cambuse 2 Callistrate Mars 8 Citronelle Bijou 17 Rose De Mai (1900) BLAIR ATHOL 10 Silvio Silverhair 1 May Pole Knight Of The Garter 3 Merry May May Queen 11 -

Chef-De-Race List
Chefs-de-Race as of October 2019 (listed alphabetically) ABERNANT (B) DJEBEL (I) MONSUN (C/S) RUN THE GANTLET (P) ACK ACK (I/C) DONATELLO II (P) MONTJEU (C/S) SADLER'S WELLS (C/S) ADMIRAL DRAKE (P) DOUBLE JAY (B) MOSSBOROUGH (C) SARDANAPALE (P) ALCANTARA II (P) DR. FAGER (I) MR. PROSPECTOR (B/C) SEA-BIRD (S) ALIBHAI (C) DUBAWI (I/S) MY BABU (B) SEATTLE SLEW (B/C) ALIZIER (P) EIGHT THIRTY (I) NASHUA (I/C) SECRETARIAT (I/C) ALYCIDON (P) EL PRADO (B/I) NASRULLAH (B) SHAMARDAL (I/C) ALYDAR (C) ELA-MANA-MOU (P) NATIVE DANCER (I/C) SHARPEN UP (B/C) APALACHEE (B) EQUIPOISE (I/C) NAVARRO (C) SHIRLEY HEIGHTS (C/P) A.P. INDY (I/C) EXCLUSIVE NATIVE (C) NEARCO (B/C) SICAMBRE (C) ASTERUS (S) FAIR PLAY (S/P) NEVER BEND (B/I) SIDERAL (C) AUREOLE (C) FAIR TRIAL (B) NEVER SAY DIE (C) SIR COSMO (B) AWESOME AGAIN (I/C) FAIRWAY (B) NIJINSKY II (C/S) SIR GALLAHAD III (C) BACHELOR’S DOUBLE (S) FAPPIANO (I/C) NINISKI (C/P) SIR GAYLORD (I/C) BAHRAM (C) FASLIYEV (B) NODOUBLE (C/P) SIR IVOR (I/C) BALDSKI (B/I) FORLI (C) NOHOLME II (B/C) SMART STRIKE (I/C) BALLYMOSS (S) FOXBRIDGE (P) NORTHERN DANCER (B/C) SOLARIO (P) BAYARDO (P) FULL SAIL (I) NUREYEV (C) SON-IN-LAW (P) BEN BRUSH (I) GAINSBOROUGH (C) OLEANDER (S) SPEAK JOHN (B/I) BEST TURN (C) GALILEO (C/S) OLYMPIA (B) SPEARMINT (P) BIG GAME (I) GALLANT MAN (B/I) ORBY (B) SPY SONG (B) BLACK TONEY (B/I) GIANT'S CAUSEWAY (C) ORTELLO (P) STAGE DOOR JOHNNY (S/P) BLANDFORD (C) GONE WEST (I/C) PANORAMA (B) STAR KINGDOM (I/C) BLENHEIM II (C/S) GRAUSTARK (C/S) PERSIAN GULF (C) STAR SHOOT (I) BLUE LARKSPUR (C) GREY DAWN II (B/I) PETER PAN (B) SUNNY BOY (P) BLUSHING GROOM (B/C) GREY SOVEREIGN (B) PETITION (I) SUNSTAR (S) BOIS ROUSSEL (S) GUNDOMAR (C) PHALARIS (B) SWEEP (I) BOLD BIDDER (I/C) HABITAT (B) PHARIS II (B) SWYNFORD (C) BOLD RUCKUS (I/C) HAIL TO REASON (C) PHAROS (I) T.V. -

2020 International List of Protected Names
INTERNATIONAL LIST OF PROTECTED NAMES (only available on IFHA Web site : www.IFHAonline.org) International Federation of Horseracing Authorities 03/06/21 46 place Abel Gance, 92100 Boulogne-Billancourt, France Tel : + 33 1 49 10 20 15 ; Fax : + 33 1 47 61 93 32 E-mail : [email protected] Internet : www.IFHAonline.org The list of Protected Names includes the names of : Prior 1996, the horses who are internationally renowned, either as main stallions and broodmares or as champions in racing (flat or jump) From 1996 to 2004, the winners of the nine following international races : South America : Gran Premio Carlos Pellegrini, Grande Premio Brazil Asia : Japan Cup, Melbourne Cup Europe : Prix de l’Arc de Triomphe, King George VI and Queen Elizabeth Stakes, Queen Elizabeth II Stakes North America : Breeders’ Cup Classic, Breeders’ Cup Turf Since 2005, the winners of the eleven famous following international races : South America : Gran Premio Carlos Pellegrini, Grande Premio Brazil Asia : Cox Plate (2005), Melbourne Cup (from 2006 onwards), Dubai World Cup, Hong Kong Cup, Japan Cup Europe : Prix de l’Arc de Triomphe, King George VI and Queen Elizabeth Stakes, Irish Champion North America : Breeders’ Cup Classic, Breeders’ Cup Turf The main stallions and broodmares, registered on request of the International Stud Book Committee (ISBC). Updates made on the IFHA website The horses whose name has been protected on request of a Horseracing Authority. Updates made on the IFHA website * 2 03/06/2021 In 2020, the list of Protected -

2008 International List of Protected Names
LISTE INTERNATIONALE DES NOMS PROTÉGÉS (également disponible sur notre Site Internet : www.IFHAonline.org) INTERNATIONAL LIST OF PROTECTED NAMES (also available on our Web site : www.IFHAonline.org) Fédération Internationale des Autorités Hippiques de Courses au Galop International Federation of Horseracing Authorities _________________________________________________________________________________ _ 46 place Abel Gance, 92100 Boulogne, France Avril / April 2008 Tel : + 33 1 49 10 20 15 ; Fax : + 33 1 47 61 93 32 E-mail : [email protected] Internet : www.IFHAonline.org La liste des Noms Protégés comprend les noms : The list of Protected Names includes the names of : ) des gagnants des 33 courses suivantes depuis leur ) the winners of the 33 following races since their création jusqu’en 1995 first running to 1995 inclus : included : Preis der Diana, Deutsches Derby, Preis von Europa (Allemagne/Deutschland) Kentucky Derby, Preakness Stakes, Belmont Stakes, Jockey Club Gold Cup, Breeders’ Cup Turf, Breeders’ Cup Classic (Etats Unis d’Amérique/United States of America) Poule d’Essai des Poulains, Poule d’Essai des Pouliches, Prix du Jockey Club, Prix de Diane, Grand Prix de Paris, Prix Vermeille, Prix de l’Arc de Triomphe (France) 1000 Guineas, 2000 Guineas, Oaks, Derby, Ascot Gold Cup, King George VI and Queen Elizabeth, St Leger, Grand National (Grande Bretagne/Great Britain) Irish 1000 Guineas, 2000 Guineas, Derby, Oaks, Saint Leger (Irlande/Ireland) Premio Regina Elena, Premio Parioli, Derby Italiano, Oaks (Italie/Italia) -

2009 International List of Protected Names
Liste Internationale des Noms Protégés LISTE INTERNATIONALE DES NOMS PROTÉGÉS (également disponible sur notre Site Internet : www.IFHAonline.org) INTERNATIONAL LIST OF PROTECTED NAMES (also available on our Web site : www.IFHAonline.org) Fédération Internationale des Autorités Hippiques de Courses au Galop International Federation of Horseracing Authorities __________________________________________________________________________ _ 46 place Abel Gance, 92100 Boulogne, France Tel : + 33 1 49 10 20 15 ; Fax : + 33 1 47 61 93 32 E-mail : [email protected] 2 03/02/2009 International List of Protected Names Internet : www.IFHAonline.org 3 03/02/2009 Liste Internationale des Noms Protégés La liste des Noms Protégés comprend les noms : The list of Protected Names includes the names of : ) des gagnants des 33 courses suivantes depuis leur ) the winners of the 33 following races since their création jusqu’en 1995 first running to 1995 inclus : included : Preis der Diana, Deutsches Derby, Preis von Europa (Allemagne/Deutschland) Kentucky Derby, Preakness Stakes, Belmont Stakes, Jockey Club Gold Cup, Breeders’ Cup Turf, Breeders’ Cup Classic (Etats Unis d’Amérique/United States of America) Poule d’Essai des Poulains, Poule d’Essai des Pouliches, Prix du Jockey Club, Prix de Diane, Grand Prix de Paris, Prix Vermeille, Prix de l’Arc de Triomphe (France) 1000 Guineas, 2000 Guineas, Oaks, Derby, Ascot Gold Cup, King George VI and Queen Elizabeth, St Leger, Grand National (Grande Bretagne/Great Britain) Irish 1000 Guineas, 2000 Guineas, -

Poemsgordon00gord Bw.Pdf
itii Vi THE LIBRARY OF THE UNIVERSITY OF CALIFORNIA LOS ANGELES GIFT OF eiff U.C. Library \ \\ / / ADAM LINDSAY GORDON. POEMS BY ADAM LINDSAY GORDON. SEA SPRAY AND SMOKE DRIFT. BUSH BALLADS AND GALLOPING RHYMES. MISCELLANEOUS POEMS. ASHTAROTH : A DRAMATIC LYRIC. THE ROLL OF THE KETTLEDRUM {ILLUSTRATED). LONDON: ROBT. A. THOMPSON & CO., 8 Farringdon Avenue, Farringdon Street, E.G. Melbourne: A. H. Massina & Co. 1895- THE LIBRARY OF THE UNIVERSITY OF CALIFORNIA LOS ANGELES GIFT OF [Eni, Gift U.C. Library 3n flDemoriam. (A. L. GORDON.) At rest ! Hard by the margin of that sea Whose sounds are mingled with his noble verse, Now lies the shell that never more will house The fine, strong spirit of my gifted friend. Yea, he who flashed upon us suddenly, A shining soul with syllables of fire, Who sang the first great songs these lands can claim To be their own ; the one who did not seem To know what royal place awaited him Within the Temple of the Beautiful, Has passed away ; and we who knew him, sit Aghast in darkness, dumb with that great grief, Whose stature we cannot yet comprehend ; While over yonder churchyard, hearsed with pines, The night-wind sings its immemorial hymn, And sobs above a newly-covered grave. The bard, the scholar, and the man who lived That frank, that open-hearted life which keeps The splendid fire of English chivalry From out the one dying ; who never wronged A fellow-man the faithful ; friend who judged The many, anxious to be loved of him. By what he saw, and not by what he heard. -

Battleship 1927 Stallion
TesioPower jadehorse Battleship () Melbourne 1 WEST AUSTRALIAN Mowerina 7 Australian Young Emilius 6 Emilia Persian 11 Spendthrift (1876) Boston (RH) Lexington (RH) Alice Carneal (RH) Aerolite (rh) Glencoe 1 Florine (RH) Melody (RH) Hastings (1893) Kingston 12 Blue Mantle Paradigm 1 Blue Ruin Alarm 19 Raffle The Swede 1 Cinderella (1885) Weatherbit 12 Brown Bread Brown Agnus 16 Manna Birdcatcher 11 Tartlet Don John Mare 21 Fair Play (1905) The Baron 24 STOCKWELL Pocahontas 3 Doncaster Teddington 2 Marigold Ratan Mare 5 TADCASTER (1877) Touchstone 14 NEWMINSTER Beeswing 8 Clemence Euclid 7 Eulogy Martha Lynn 2 Fairy Gold (1896) Vedette 19 GALOPIN Flying Duchess 3 GALLIARD MACARONI 14 Mavis Beau Merle 13 Dame Masham (1889) NEWMINSTER 8 HERMIT Seclusion 5 Pauline Brother To Strafford 8 Lady Masham Maid Of Masham 9 Man O' War (1917) STOCKWELL 3 St Albans Bribery 2 Springfield Marsyas 12 Viridis Maid Of Palmyra 12 Sainfoin (1887) LORD CLIFDEN 2 Wenlock Mineral 4 Sanda STOCKWELL 3 Sandal Lady Evelyn 2 Rock Sand (1900) Vedette 19 GALOPIN Flying Duchess 3 St Simon KING TOM 3 St Angela Adeline 11 Roquebrune (1893) NEWMINSTER 8 HERMIT Seclusion 5 St Marguerite STOCKWELL 3 Devotion Alcestis 4 Mahubah (1910) NEWMINSTER 8 LORD CLIFDEN The Slave 2 Hampton Kettledrum 3 Lady Langden Haricot 10 Merry Hampton (1884) STOCKWELL 3 Broomielaw Queen Mary 10 Doll Tearsheet Longbow 21 Mrs Quickly Venus 22 Merry Token (1891) Sweetmeat 21 MACARONI Jocose 14 Macgregor The Fallow Buck 6 Necklace Bracelet 4 Mizpah (1880) The Cure 6 Underhand Contraction 43 Mare By -

Two Day Sporting Memorabilia - Day 1 Monday 05 November 2012 12:30
Two Day Sporting Memorabilia - Day 1 Monday 05 November 2012 12:30 Graham Budd Auctions Ltd Sotheby's 34-35 New Bond Street London W1A 2AA Graham Budd Auctions Ltd (Two Day Sporting Memorabilia - Day 1) Catalogue - Downloaded from UKAuctioneers.com Lot: 1 took full use of the opportunity A gold model of a kettledrum steering Reefer to a comfortable commemorating the victory of the 3 lengths win. Rossmore was racehorse 'Kettledrum' in the sure of victory and no doubt a 1861 Derby, the 'drumskin' made healthy wager helped pay for the with a grey hardstone and being purchase of this grand cup to a seal stamp with the following more than suitably commemorate hand written inscription reverse his horse's achievement. engraved KETTLEDRUM, Estimate: £6,000.00 - £8,000.00 WINNER OF THE DERBY, 1861, attached to a gold chain nearly 12in. in length Lot: 3 Estimate: £800.00 - £1,200.00 A preserved hoof of 'Zoedone' the 1883 Grand National winner, mounted with electroplate and Lot: 2 converted as an inkwell, the A magnificent trophy hinged lid inscribed ZOEDONE, commemorating the victory of GRAND NATIONAL WINNER, Lord Rossmore's 'Reefer' in the 1883, PRINCE KARL KINSKY 1882 Nottingham Spring Estimate: £400.00 - £600.00 Handicap, in hallmarked continental silver, a large vase & cover, the handles formed by Lot: 4 swan necks, the body with a A pair of royally presented racing band featuring winged horses plates worn by The Prince of and laurel, the square base with Wales's racehorse Persimmon ball & claw feet and inscribed and his full brother Diamond