PDF Generation Date :- Thu, 02 Sep 2021 08:13:27
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Rohan Prithvii, Ghatkopar West, Lal Bahadur Shastri Marg, Mumbai Fact Sheet
Rohan Prithvii, Ghatkopar West, Lal Bahadur Shastri Marg, Mumbai Fact Sheet Brochure Rohan Prithvii, Mumbai Brochure may be downloaded from the link: http://zrks.in/230169e6 Overview Lifescapes Prithvii is designed to marry luxurious living spaces within to scenic natural beauty outside. The project is meant for people who like space. The Project is tucked away off LBS Marg in Ghatkopar West. Close to the project, at a 3-minute drive, is the upcoming Asalfa Metro Station. So, once the Versova-Andheri-Ghatkopar Metro is operational, you can hope to reach the Western Suburbs in approximately 20 minutes. Project USP A completely gated community that promotes a sense of community living along with privacy Project provides all the basic amenities that are required to meet all your needs 3-tiered stringent security measures to ensure the safety of all students Covered parking area with reserved spaces for all residents Separate parking spaces for all guests and visitors Several civic amenities such as premium schools, medical centers and hospitality outlets in the neighbourhood Lush green surroundings and exquisite landscaping around the residential units 100 % power back up for building and all common facilities to ensure smooth and uninterrupted running of the same Location Advantages Located at Ghatkopar, Lal Bahadur Shastri Marg, Mumbai Easy connectivity to major areas of city Major schools and hospitals situated at a distance of 5 km Schools and Universities are in proximity to the society Facts Builder Name Rohan Lifescapes Price Range 1.25 Cr to 3.10 Cr Size Range 1315 - 2052 Sq.Ft Property Type Apartment Flat Type , 2 BHK , 3 BHK Project Status Under Construction Possesion Date Quarter 4 2018 Project Span 2 Acres No of Towers 4 Total No of Units 177 Unit Details Property Type Unit Type Saleable Area Floor Plan Apartment 2 BHK 1315 Sq. -
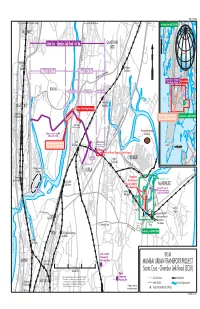
Chembur Link Road (SCLR) Matunga to Mumbai Rail Station This Map Was Produced by the Map Design Unit of the World Bank
IBRD 33539R To Jogeswari-Virkhroli Link Road / Borivali To Jogeswari-Virkhroli Link Road To Thane To Thane For Detail, See IBRD 33538R VILE PARLE ek re GHATKOPAR C i r Santa Cruz - Chembur Link Road: 6.4 km o n a SATIS M Mumbai THANE (Kurla-Andhai Road) Vile Parle ek re S. Mathuradas Vasanji Marg C Rail alad Station Ghatkopar M Y Rail Station N A Phase II: 3.0 km Phase I: 3.4 km TER SW S ES A E PR X Lal Bahadur Shastri Marg WESTERN EXPRESSWAY E Santa Cruz - Chembur Link Road: 6.4 km Area of Map KALINA Section 1: 1.25 km Section 2: 1.55 km Section 3: .6 km ARABIAN Swami Vivekananda Marg SEA Vidya Vihar Thane Creek SANTA CRUZ Rail Station Area of Gazi Nagar Request Mahim Bay Santa Cruz Rail Station Area of Shopkeepers' Request For Detail, See IBRD 33540R For Detail, See IBRD 33314R MIG Colony* (Middle Income Group) Central Railway Deonar Dumping 500m west of river and Ground 200m south of SCLR Eastern Expressway R. Chemburkar Marg Area of Shopkeepers' Request Kurla MHADA Colony* CHURCHGATE CST (Maharashtra Housing MUMBAI 012345 For Detail, See IBRD 33314R Rail Station and Area Development Authority) KILOMETERS Western Expressway Area of Bharathi Nagar Association Request S.G. Barve Marg (East) Gha Uran Section 2 Chembur tko CHEMBUR Rail Station parM ankh urdLink Bandra-Kurla R Mithi River oad To Vashi Complex KURLA nar Nala Deo Permanent Bandra Coastal Regulation Zones Rail Station Chuna Batti Resettlement Rail Station Housing Complex MANKHURD at Mankhurd Occupied Permanent MMRDA Resettlement Housing Offices Govandi Complex at Mankhurd Rail Station Deonar Village Road Mandala Deonarpada l anve Village P Integrated Bus Rail Sion Agarwadi Interchange Terminal Rail Station Mankhurd Mankhurd Correction ombay Rail Station R. -

Details of Shareholders' Dividend Outstanding for 7
NELCO LIMITED LIST OF SHAREHOLDERS WHOSE DIVIDEND IS OUTSTANDING FOR 7 CONSECUTIVE YEARS ( 2013 & 2019 ) Total Outstanding SrNo Folio No/DPID ClientID 1st Holder Name 1st Joint Holder 2nd Joint Holder Add Line 1 Add Line 2 Add Line 3 Add Line 4 Add City Add Pincode Current Holding Amount_24JAN13 Amount_26JUL19 Amount Rs. 1 NED0000022 DENIS J MATHIAS C/O MR E J MATHIAS NO 1 WALTON ROAD BANGALORE 0 100 200.00 50.00 150.00 2 NEH0000053 HEMANT PREMJI THACKER Mr Premji Valji Thacker 7/34 SHYAM NIVAS B DESAI ROAD 0 150 300.00 75.00 225.00 3 NEJ0000046 JAYENDRAKUMAR KUNJBIHARILAL VYAS MAHATMA GANDHI ROAD DOHAD 0 50 100.00 25.00 75.00 4 NEK0000107 K T NITHYANANDAN PEOPLE S RADIO SERVICE TELLICHERRY 0 40 80.00 20.00 60.00 5 NEP0000016 PHIROZE DARASHAW ELAVIA STATION ROAD RAJPIPLA 0 10 20.00 5.00 15.00 6 NES0000132 S M S INVESTMENT CORPN PVT LTD BRANCH OFFICE RAJMAHAL PALACE JAIPUR 0 100 200.00 50.00 150.00 7 NEI0000004 TATA INVESTMENT CORPORATION LIMITED EWART HOUSE BRUCE STREET FORT BOMBAY 1 350 700.00 175.00 525.00 8 NEA0010207 AADITYA GARG C/O ANIL KUMAR GUPTA 164-D KAMLA NAGAR DELHI 110007 50 100.00 25.00 75.00 9 NES0010358 SANCHIT GUPTA C/O ANIL KUMAR GUPTA 164-D KAMLA NAGAR DELHI 110007 50 100.00 25.00 75.00 10 IN30177416703637 SHWETA KAPOOR 12/2 SHAKTI NAGAR DELHI 110007 100 200.00 50.00 150.00 11 IN30046810025744 ANUPMA SUNEJA Sameer Suneja C-5/20 II ND FLOOR SAFDURJUNG DEVELOPMENT AREA NEW DELHI 110016 200 400.00 100.00 300.00 12 NEA0000117 ATARKALI JAIN S 322 PANCHSHILA PARK NEW DELHI 110017 110017 50 100.00 25.00 75.00 13 NER0011096 -

TCS/SE/73/2021-22 July 23, 2021 National Stock Exchange of India
TCS/SE/73/2021-22 July 23, 2021 National Stock Exchange of India Limited BSE Limited Exchange Plaza, Bandra Kurla Complex, P. J. Towers, Dalal Street, Mumbai-400051 Mumbai-400001 Symbol: TCS Scrip Code No. 532540 (BSE) Dear Sirs, Sub: Intimation for loss of share certificate as per Regulation 39(3) of SEBI (Listing Obligations and Disclosure Requirements) Regulations, 2015 (“Listing Regulations”) Pursuant to Regulation 39(3) of Listing Regulations, please find enclosed the intimation letter received from our Registrars and Share Transfer Agent - TSR Darashaw Consultants Private Limited, providing information regarding loss of share certificates received from the shareholder. This is for your information and records. Thanking you, Yours faithfully, For Tata Consultancy Services Limited Rajendra Moholkar Company Secretary Encl: As above 9th Floor Nirmal Building Nariman Point Mumbai 400 021 Tel 91 22 6778 9595 Fax 91 22 6630 3672 e-mail [email protected] website www.tcs.com Registered Office 9th Floor Nirmal Building Nariman Point Mumbai 400 021 Corporate Identity No. (CIN): L22210MH1995PLC084781 \ llll&;iDARASHA\N Total .So!utione Repository REF NO.: 89 22-07-2021 TATA CONSULTANCY SERVICES LIMITED NIRMAL BUILDING, 9TH FLOOR NARIMAN POINT MUMBAI MAHARASHTRA INDIA 400021 Dear Sir(s]/Madam, UNIT : TATA CONSULTANCY SERVICES LIMITED. RE : LOSS OF SHARE CERTIFICATES. We have to advise you to put the appended Notice rega::-ding loss of Certificate [s] for attention of th"e Members of the Exchange, with instructions that they com~unicate to us immediately if they are in a position to give us information relating to any transaction or ~hereabouts of the original certificate[s]. -

Raheja Plaza, Lal Bahadur Shastri Marg, Ghatkopar West, Mumbai, India
Raheja Plaza, Lal Bahadur Shastri Marg, Ghatkopar West, Mumbai, India View this office online at: https://www.newofficeasia.com/details/serviced-offices-raheja-plaza-lal-bahad ur-shastri-marg-ghatkopar-west-mumba This modern business centre provides a fully furnished and well-equipped business centre which benefits from an array of facilities and professional support. With glass partitions and large windows, this centre offers a bright and airy atmosphere that is designed to enhance motivation while boasting high speed internet access, VOIP telephony and photocopying technology. Executive meeting rooms are available on request and provide state-of-the-art videoconferencing technology which is supported by a team of friendly staff who provide IT support, secretarial services and mail handling duties that save you time and energy. Transport links Nearest tube: Ghatkopar Nearest railway station: Ghatkopar, Vikhroli Nearest road: Ghatkopar Nearest airport: Ghatkopar Key features 24 hour access Administrative support Board room Car parking spaces Cat 6 networking or higher Conference rooms Furnished workspaces Hot desking IT support available Lift Meeting rooms Office cleaning service Photocopying available Postal facilities/mail handling Reception staff Video conference facilities Voicemail VOIP telephony WC (separate male & female) Wireless networking Location Situated within a thriving commercial community, there is a wealth of neighbouring office buildings, showrooms and complexes which offer excellent networking opportunities to help your business flourish. A diverse selection of top-end restaurants and hotels also reside nearby of provide a more informal setting for entertaining potential clients. Despite belonging to a bustling professional hub, tranquil expanses of landscape and the beautiful coast reside on the other side of the Eastern Express Highway while both train and metro stations are a 5 minute drive away. -

TERRORISM, COMMUNAL POLITICS and ETHNIC DEMOGRAPHY: IS THERE a CAUSAL CONNECTION? Empirical Analysis of Terrorist Incidents in Maharashtra
M. Mayilvaganan TERRORISM, COMMUNAL POLITICS AND ETHNIC DEMOGRAPHY: IS THERE A CAUSAL CONNECTION? Empirical Analysis of Terrorist Incidents in Maharashtra May 2020 International Strategic and Security1 Studies Programme National Institute of Advanced Studies Bengaluru, India Research Report NIAS/CSS/ISSSP/U/RR/08/2020 TERRORISM, COMMUNAL POLITICS AND ETHNIC DEMOGRAPHY: IS THERE A CAUSAL CONNECTION? Empirical Analysis of Terrorist Incidents in Maharashtra National Institute of Advanced Studies Bengaluru, India 2020 © National Institute of Advanced Studies, 2020 Published by National Institute of Advanced Studies Indian Institute of Science Campus Bengaluru – 560012 Tel: 22185000, Fax: 22185028 Email: [email protected] NIAS Report: NIAS/CSS/ISSSP/U/RR/08/2020 ISBN 978-93-83566-38-9 Content Introduction .............................................................................. 1 India and Terrorism .................................................................. 7 Maharashtra ............................................................................ 8 Micro Level Analysis ................................................................. 10 Key Observations ..................................................................... 24 Inference ................................................................................. 26 INTRODUCTION Is there a causal link between ethnic demography, communal violence, local politics and terrorism? What factors might prompt a terrorist to choose a target place? Why the states like Maharashtra -

Where Scenic Lake View Homes Marry a Grand Investment Plan
Where scenic lake view homes marry a grand investment plan. Introducing Information Memorandum www.Zricks.com Page 1 Powai is a suburban neighbourhood located in the north-east of Mumbai, and EVER-ALLURING is situated on the banks of the beautiful Powai Lake. In the last few years, POWAI: Powai has changed from a scenic lakeside picnic spot; to one of the fastest developing suburbs. It has grown prolifically and exponentially to become one of Mumbai's most sought after commercial and residential hubs. With an integrated residential township and many residential complexes in and around its vicinity, Powai is home to a number of commercial and educational institutions such as ICICI Bank, SBI, HSBC and HDFC Bank, Podar International School, Bombay Scottish School, ICFAI Business School and IIT Bombay. Luxury hotels, such as Renaissance Hotel, Radisson Blu Plaza and Ramada Powai and mega stores like D Mart and Haiko Supermarket are also a part of the vicinity. Corporate offices such as Deloitte, CRISIL and Bayer are in Powai too. All of this makes Powai a great locality to find appreciable value in. www.Zricks.com Page 2 INFRASTRUCTURE The Mumbai Development Plan of 1991 was instrumental in bringing about key infrastructure projects for decongesting the city center. Consequently, the city’s DEVELOPMENT IN POWAI eastern suburbs such as Powai, benefited immensely from these policy measures. HAS GONE FROM Due to the reduced commuting time to South Mumbai, demand for commercial and residential properties along with increase in housing prices in locations such STRENGTH TO STRENGTH: as Powai has increased by approximately 28–30 per cent over the last two years. -

CIN : L24100MH1969PLC014336 10Th June, 2021 the Secretary The
10th June, 2021 The Secretary The Secretary BSE Ltd. National Stock Exchange of India Ltd. Corporate Relationship Dept., Exchange Plaza, Plot no. C/1, G Block, 14th floor, P. J. Tower, Bandra-Kurla Complex, Dalal Street, Fort Bandra (E), Mumbai - 400 001 Mumbai - 400 051 Stock Code – 500331 Stock Code - PIDILITIND Dear Sir, Sub: Intimation as per Regulation 39(3) of the SEBI (Listing Obligations and Disclosure Requirements) Regulations, 2015 Please find enclosed the intimation letter dated 10th June, 2021, received from our RTA - M/s. TSR Darashaw Consultants Private Limited, providing information regarding loss of share certificates of the shareholder(s) of the Company. This information is being submitted pursuant to Regulation 39(3) of the SEBI (Listing Obligations and Disclosure Requirements) Regulations, 2015. Kindly take the same on your records. Thanking You, Yours faithfully, For Pidilite Industries Limited Puneet Bansal Company Secretary Encl. as above CIN : L24100MH1969PLC014336 REF NO.: 39 10-06-2021 National Stock Exchange of India Ltd. Exchange Plaza Plot No.c-1, G-Block IFB Centre Bandra-Kurla Complex Bandra (East), Mumbai 400051 Maharashtra India Attn : The Secretary of Stock Exchange Dear Sir[s]/Madam, UNIT : PIDILITE INDUSTRIES LIMITED. RE : LOSS OF SHARE CERTIFICATES. ----------------------------------------------------- We have to advise you to put the appended Notice regarding loss of Certificate[s] for attention of the Members of the Exchange, with instructions that they communicate to us immediately if they are in a position to give us information relating to any transaction or whereabouts of the original certificate[s]. Yours faithfully, for TSR DARASHAW CONSULTANTS PRIVATE LIMITED. This is computer generated letter and does not require signature. -

A-494 Bus Time Schedule & Line Route
A-494 bus time schedule & line map A-494 Mulund Depot - Reti Bunder Circle View In Website Mode The A-494 bus line (Mulund Depot - Reti Bunder Circle) has 2 routes. For regular weekdays, their operation hours are: (1) Mulund Depot: 6:42 AM - 11:12 AM (2) Reti Bunder Circle: 7:30 AM - 10:30 AM Use the Moovit App to ƒnd the closest A-494 bus station near you and ƒnd out when is the next A-494 bus arriving. Direction: Mulund Depot A-494 bus Time Schedule 39 stops Mulund Depot Route Timetable: VIEW LINE SCHEDULE Sunday 6:42 AM - 11:12 AM Monday 6:42 AM - 11:12 AM Reti Bunder Circle Mumbai - Pune Road, Thāne Tuesday 6:42 AM - 11:12 AM Sainath Nagar Wednesday 6:42 AM - 11:12 AM Subhash Tower Thursday 6:42 AM - 11:12 AM Friday 6:42 AM - 11:12 AM Vastu Anand Saturday 6:42 AM - 11:12 AM Parsik Nagar Raj Park Prem Nagar A-494 bus Info Direction: Mulund Depot Parsik Shiv Mandir Stops: 39 Trip Duration: 27 min Kharegaon Naka Line Summary: Reti Bunder Circle, Sainath Nagar, Subhash Tower, Vastu Anand, Parsik Nagar, Raj Park, Prem Nagar, Parsik Shiv Mandir, Kharegaon Kharegaon Dattawadi Naka, Kharegaon Dattawadi, Sukur Park, Sahyadri Society, Manisha Nagar, Kalwa Naka / Shivaji Chowk, Sukur Park Court Naka, R.T.O.O∆ce (Thane), Thane Civil Hospital, Thane Civil Hospital, Shishu Gyan Mandir, Sahyadri Society New Shakti Mill, Khopat S.T.Stand (Thane), Makhamali Talao, Vandana Cinema / Talkies, Hari Manisha Nagar Niwas, Hari Nivas Circle, Marathon Chowk (Teen Hath Naka), Marathon Chowk (Teen Hath Naka), Kalwa Naka / Shivaji Chowk Johnson Company, Wagle -

TCS/SE/66/2021-22 July 7, 2021 National Stock Exchange of India
TCS/SE/66/2021-22 July 7, 2021 National Stock Exchange of India Limited BSE Limited Exchange Plaza, Bandra Kurla Complex, P. J. Towers, Dalal Street, Mumbai-400051 Mumbai-400001 Symbol: TCS Scrip Code No. 532540 (BSE) Dear Sirs, Sub: Intimation for loss of share certificate as per Regulation 39(3) of SEBI (Listing Obligations and Disclosure Requirements) Regulations, 2015 (“Listing Regulations”) Pursuant to Regulation 39(3) of Listing Regulations, please find enclosed the intimation letter received from our Registrars and Share Transfer Agent - TSR Darashaw Consultants Private Limited, providing information regarding loss of share certificates received from the shareholder. This is for your information and records. Thanking you, Yours faithfully, For Tata Consultancy Services Limited Rajendra Moholkar Company Secretary Encl: As above 9th Floor Nirmal Building Nariman Point Mumbai 400 021 Tel 91 22 6778 9595 Fax 91 22 6630 3672 e-mail [email protected] website www.tcs.com Registered Office 9th Floor Nirmal Building Nariman Point Mumbai 400 021 Corporate Identity No. (CIN): L22210MH1995PLC084781 [l; DARASHA\N T otal Solutions Repository REF NO.: 87 06-07- 2021 TATA CONSULTANCY SERVICES LIMITED NIRMAL BUILDING, 9TH FLOOR NARIMAN PO INT MUMBAI MAHARASHTRA INDIA 400021 Dear Sir[s ) / Madam, UNIT : TATA CONSULTANCY SERVICES LIMITED. RE : LOSS OF SHARE CERTIFICATES . We have to advise you to put the appended Notice regarding loss of Certificate[s) for attention of the Members of the Exchange, with instructions that they communicate to us immediately if they are in a position to give us information relating to any transacti on or whereabouts of the original certificate[s) . -

RECONCILIATION of SHARE CAPITAL AUDIT Stock Exchange
RECONCILIATION OF SHARE CAPITAL AUDIT Scrip code* 509470 NSE Symbol NA MSEI Symbol NA ISIN INE01TL01014 Name of the company* BOMBAY OXYGEN INVESTMENTS LIMITED Registered office address Registered office address* 22/B, Mittal Tower, B Wing, Nariman Point Registered office state* Maharashtra Registered office city* Mumbai Registered office district* Mumbai Registered office pin code* 400021 ISD Code* STD Code* Number* Registered office contact number* +91 022 66107503 Registered office fax 022 66107512 Registered office country* INDIA Registered office website www.bomoxy.com Registered office email [email protected] Correspondence address Same as above Yes Correspondence address 22/B, Mittal Tower, B Wing, Nariman Point Correspondence state Maharashtra Correspondence city Mumbai Correspondence district Mumbai Correspondence pin code 400021 ISD Code STD Code Number Correspondence contact number +91 022 66107503 Correspondence fax 022 66107512 Correspondence country INDIA Correspondence email [email protected] Reporting quarter* 31-03-2021 Face value* 100 Name of stock Listed % Of total issued Stock Exchange Details : Exchange Capital capital Name of other stock exchanges where the company's BSE Ltd 150000 100 securities are listed Remarks Capital Details : Number of shares % Of total issued capital Issued capital* 150000 Listed capital (BSE) (As per company records)* 150000 100 Held in dematerialised form in CDSL* 120982 80.65 Held in dematerialised form in NSDL* 13007 8.67 Physical* 16011 10.67 Total no.of shares* 150000 100 Reasons for difference if any, Between issued capital and listed capital* 0 Reasons for difference if any, Between issued capital and total number of shares* 0 Reasons for difference if any, Between listed capital and total number of shares* 0 Certifying the details of changes in share capital during the quarter under consideration as per Table below : Whether changes during the quarter* No Register of members is updated* Yes If not, Updated upto which date Reference of previous quarter with regards to excess dematerialised shares,If any. -

Mahindra Splendour, Bhandup West, Lal Bahadur Shastri Marg, Mumbai Fact Sheet
Mahindra Splendour, Bhandup West, Lal Bahadur Shastri Marg, Mumbai Fact Sheet Brochure Mahindra Splendour, Mumbai Brochure may be downloaded from the link: http://zrks.in/95ff589e Overview Mahindra Splendour is a residential project located at Bhandup West, Lal Bahadur Shastri Marg, Mumbai. It is a unique project which offers 2 and 3 BHK homes and completes the package with a number of useful features and amenities for all residents. These apartments are meticulously designed along with the rest of the project site.Mumbai is one of the hotspots for residential development and therefore has a lot of growth potential. Mahindra Splendour is backed by the Mahindra Lifespace Developers Ltd. one of the most trusted real estate developers in India, and is therefore a prime choice for a wholesome life experience. Your home is your world. It should be everything you want it to be. An embodiment of you and the way you think: modern, forward-looking and conscientious.Mahindra Splendour is just such a home. One which goes beyond the tangible to offer you the intangible luxury of living in complete harmony with nature, and one step closer to the future. Project USP A completely gated community that promotes a sense of community living along with privacy Project provides all the basic amenities that are required to meet all your needs 3-tiered stringent security measures to ensure the safety of all students Covered parking area with reserved spaces for all residents Separate parking spaces for all guests and visitors Several civic amenities such as