Assembly Bill No. 2309 Passed the Assembly April 24, 2014 Chief
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Controlled Substances Included. [M.S.A
1998 PUBLIC AND LOCAL ACTS [No. 319] (HB 4065) AN ACT to amend 1978 PA 368, entitled “An act to protect and promote the public health; to codify, revise, consolidate, classify, and add to the laws relating to public health; to provide for the prevention and control of diseases and disabilities; to provide for the classification, administration, regulation, financing, and maintenance of personal, environ- mental, and other health services and activities; to create or continue, and prescribe the powers and duties of, departments, boards, commissions, councils, committees, task forces, and other agencies; to prescribe the powers and duties of governmental entities and officials; to regulate occupations, facilities, and agencies affecting the public health; to regulate health maintenance organizations and certain third party administrators and insurers; to provide for the imposition of a regulatory fee; to promote the efficient and economical delivery of health care services, to provide for the appropriate utilization of health care facilities and services, and to provide for the closure of hospitals or consolidation of hospitals or services; to provide for the collection and use of data and information; to provide for the transfer of property; to provide certain immunity from liability; to regulate and prohibit the sale and offering for sale of drug paraphernalia under certain circumstances; to provide for penalties and remedies; to provide for sanctions for violations of this act and local ordinances; to repeal certain acts and parts of acts; to repeal certain parts of this act; and to repeal certain parts of this act on specific dates,” by amending sections 7218 and 7401 (MCL 333.7218 and 333.7401), section 7401 as amended by 1996 PA 249, and by adding section 7401a. -

Proposed Regulation of the State Board of Pharmacy
PROPOSED REGULATION OF THE STATE BOARD OF PHARMACY LCB File No. R133-14 Workshop July 24, 2014 NAC 453.540 Schedule IV. (NRS 453.146, 639.070) 1. Schedule IV consists of the drugs and other substances listed in this section, by whatever official, common, usual, chemical or trade name designated. 2. Unless specifically excepted or unless listed in another schedule, any material, compound, mixture or preparation containing any of the following narcotic drugs, including, without limitation, their salts, calculated as the free anhydrous base of alkaloid, is hereby enumerated on schedule IV, in quantities: (a) Not more than 1 milligram of difenoxin and not less than 25 micrograms of atropine sulfate per dosage unit; or (b) Dextropropoxyphene (alpha-(+)-4-dimethylamino-1,2-diphenyl-3-methyl-2-propionoxy- butane). 3. Unless specifically excepted or unless listed in another schedule, any material, compound, mixture or preparation which contains any quantity of the following substances, including, without limitation, their salts, isomers and salts of isomers, is hereby enumerated on schedule IV, whenever the existence of such salts, isomers and salts of isomers is possible within the specific chemical designation: Alprazolam; Barbital; Bromazepam; Butorphanol; Camazepam; Carisoprodol; Chloral betaine; Chloral hydrate; Chlordiazepoxide; Clobazam; Clonazepam; Clorazepate; Clotiazepam; Cloxazolam; Delorazepam; Diazepam; Dichloralphenazone; Estazolam; Ethchlorvynol; Ethyl loflazepate; Fludiazepam; Flunitrazepam; --1-- Agency Draft of Proposed Regulation R133-14 Flurazepam; Halazepam; Haloxazolam; Ketazolam; Loprazolam; Lorazepam; Lormetazepam; Mebutamate; Medazepam; Meprobamate; Methohexital; Methylphenobarbital (mephobarbital); Midazolam; Nimetazepam; Nitrazepam; Nordiazepam; Oxazepam; Oxazolam; Paraldehyde; Petrichloral; Phenobarbital; Pinazepam; Prazepam; Quazepam; Tramadol (2-((dimethylamino)methyl)-1-(3-methoxyphenyl)cyclohexanol) Temazepam; Tetrazepam; Triazolam; Zaleplon; Zolpidem; or Zopiclone. -

124.210 Schedule IV — Substances Included. 1
1 CONTROLLED SUBSTANCES, §124.210 124.210 Schedule IV — substances included. 1. Schedule IV shall consist of the drugs and other substances, by whatever official name, common or usual name, chemical name, or brand name designated, listed in this section. 2. Narcotic drugs. Unless specifically excepted or unless listed in another schedule, any material, compound, mixture, or preparation containing any of the following narcotic drugs, or their salts calculated as the free anhydrous base or alkaloid, in limited quantities as set forth below: a. Not more than one milligram of difenoxin and not less than twenty-five micrograms of atropine sulfate per dosage unit. b. Dextropropoxyphene (alpha-(+)-4-dimethylamino-1,2-diphenyl-3-methyl-2- propionoxybutane). c. 2-[(dimethylamino)methyl]-1-(3-methoxyphenyl)cyclohexanol, its salts, optical and geometric isomers and salts of these isomers (including tramadol). 3. Depressants. Unless specifically excepted or unless listed in another schedule, any material, compound, mixture, or preparation which contains any quantity of the following substances, including its salts, isomers, and salts of isomers whenever the existence of such salts, isomers, and salts of isomers is possible within the specific chemical designation: a. Alprazolam. b. Barbital. c. Bromazepam. d. Camazepam. e. Carisoprodol. f. Chloral betaine. g. Chloral hydrate. h. Chlordiazepoxide. i. Clobazam. j. Clonazepam. k. Clorazepate. l. Clotiazepam. m. Cloxazolam. n. Delorazepam. o. Diazepam. p. Dichloralphenazone. q. Estazolam. r. Ethchlorvynol. s. Ethinamate. t. Ethyl Loflazepate. u. Fludiazepam. v. Flunitrazepam. w. Flurazepam. x. Halazepam. y. Haloxazolam. z. Ketazolam. aa. Loprazolam. ab. Lorazepam. ac. Lormetazepam. ad. Mebutamate. ae. Medazepam. af. Meprobamate. ag. Methohexital. ah. Methylphenobarbital (mephobarbital). -

Revise Schedule of Controlled Substances. (Public) Sponsors: Senators J
GENERAL ASSEMBLY OF NORTH CAROLINA SESSION 2017 S 1 SENATE BILL 347* Short Title: Revise Schedule of Controlled Substances. (Public) Sponsors: Senators J. Davis, McInnis (Primary Sponsors); and Rabin. Referred to: Rules and Operations of the Senate March 22, 2017 1 A BILL TO BE ENTITLED 2 AN ACT REVISING THE SCHEDULE OF CONTROLLED SUBSTANCES TO ADD 3 SYNTHETIC FENTANYLS, DESIGNER HALLUCINOGENICS, SYNTHETIC 4 CANNABINOIDS, SYSTEM DEPRESSANTS, AND OTHER SUBSTANCES. 5 The General Assembly of North Carolina enacts: 6 SECTION 1. This act shall be known and may be cited as the "Synthetic Opioid 7 and Other Dangerous Drug Control Act." 8 SECTION 2. G.S. 90-89 reads as rewritten: 9 "§ 90-89. Schedule I controlled substances. 10 This schedule includes the controlled substances listed or to be listed by whatever official 11 name, common or usual name, chemical name, or trade name designated. In determining that a 12 substance comes within this schedule, the Commission shall find: a high potential for abuse, no 13 currently accepted medical use in the United States, or a lack of accepted safety for use in 14 treatment under medical supervision. The following controlled substances are included in this 15 schedule: 16 (1) Opiates. – Any of the following opiates, including the isomers, esters, ethers, 17 salts and salts of isomers, esters, and ethers, unless specifically excepted, or 18 listed in another schedule, whenever the existence of such isomers, esters, 19 ethers, and salts is possible within the specific chemical designation: 20 a. Acetyl-alpha-methylfentanyl 21 (N[1-(1-methyl-2-phenethyl)-4/y-piperidinyl]-N-phenylacet amide). -

Schedules of Controlled Substances (.Pdf)
PURSUANT TO THE TEXAS CONTROLLED SUBSTANCES ACT, HEALTH AND SAFETY CODE, CHAPTER 481, THESE SCHEDULES SUPERCEDE PREVIOUS SCHEDULES AND CONTAIN THE MOST CURRENT VERSION OF THE SCHEDULES OF ALL CONTROLLED SUBSTANCES FROM THE PREVIOUS SCHEDULES AND MODIFICATIONS. This annual publication of the Texas Schedules of Controlled Substances was signed by John Hellerstedt, M.D., Commissioner of Health, and will take effect 21 days following publication of this notice in the Texas Register. Changes to the schedules are designated by an asterisk (*). Additional information can be obtained by contacting the Department of State Health Services, Drugs and Medical Devices Unit, P.O. Box 149347, Austin, Texas 78714-9347. The telephone number is (512) 834-6755 and the website address is http://www.dshs.texas.gov/dmd. SCHEDULES Nomenclature: Controlled substances listed in these schedules are included by whatever official, common, usual, chemical, or trade name they may be designated. SCHEDULE I Schedule I consists of: -Schedule I opiates The following opiates, including their isomers, esters, ethers, salts, and salts of isomers, esters, and ethers, unless specifically excepted, if the existence of these isomers, esters, ethers, and salts are possible within the specific chemical designation: (1) Acetyl-α-methylfentanyl (N-[1-(1-methyl-2-phenethyl)-4-piperidinyl]-N- phenylacetamide); (2) Acetylmethadol; (3) Acetyl fentanyl (N-(1-phenethylpiperidin-4-yl)-N-phenylacetamide); (4) Acryl fentanyl (N-(1-phenethylpiperidin-4-yl)-N-phenylacrylamide) (Other name: -

Alcohol and Drug Abuse Subchapter 9 Regulated Drug Rule 1.0 Authority
Chapter 8 – Alcohol and Drug Abuse Subchapter 9 Regulated Drug Rule 1.0 Authority This rule is established under the authority of 18 V.S.A. §§ 4201 and 4202 which authorizes the Vermont Board of Health to designate regulated drugs for the protection of public health and safety. 2.0 Purpose This rule designates drugs and other chemical substances that are illegal or judged to be potentially fatal or harmful for human consumption unless prescribed and dispensed by a professional licensed to prescribe or dispense them, and used in accordance with the prescription. The rule restricts the possession of certain drugs above a specified quantity. The rule also establishes benchmark unlawful dosages for certain drugs to provide a baseline for use by prosecutors to seek enhanced penalties for possession of higher quantities of the drug in accordance with multipliers found at 18 V.S.A. § 4234. 3.0 Definitions 3.1 “Analog” means one of a group of chemical components similar in structure but different with respect to elemental composition. It can differ in one or more atoms, functional groups or substructures, which are replaced with other atoms, groups or substructures. 3.2 “Benchmark Unlawful Dosage” means the quantity of a drug commonly consumed over a twenty-four hour period for any therapeutic purpose, as established by the manufacturer of the drug. Benchmark Unlawful dosage is not a medical or pharmacologic concept with any implication for medical practice. Instead, it is a legal concept established only for the purpose of calculating penalties for improper sale, possession, or dispensing of drugs pursuant to 18 V.S.A. -

A. Schedule IV Shall Consist Of
1 REVISOR 6800.4240 6800.4240 SCHEDULE IV CONTROLLED SUBSTANCES. The following items are listed in Schedule IV: A. Schedule IV shall consist of the drugs and other substances, by whatever official name, common or usual name, chemical name, or brand name designated, listed in this part. B. Narcotic drugs. Unless specifically excepted or unless listed in another schedule, any material, compound, mixture, or preparation containing any of the following narcotic drugs, or their salts calculated as the free anhydrous base or alkaloid, in limited quantities as follows: (1) Not more than one milligram of difenoxin and not less than 25 micrograms of atropine sulfate per dosage unit. (2) Dextropropoxyphene (alpha-(+)-4-dimethylamino-1,2-diphenyl-3-methyl-2- propionoxybutane), for example, Darvon, Darvocet. C. Depressants. Unless specifically excepted or unless listed in another schedule, any material, compound, mixture, or preparation which contains any quantity of the following substances, including its salts, isomers, and salts of isomers whenever the existence of such salts, isomers, and salts of isomers is possible within the specific chemical designation: Statutory Name Some examples of common names, trade names, or names of products which contain a controlled substance (1) Alprazolam Xanax (2) Barbital Barbitone (3) Bromazepam (4) Camazepam (5) Chloral betaine Beta-Chlor (6) Chloral hydrate Noctec, Somnos (7) Chlordiazepoxide Librium, Libritabs (8) Clobazam (9) Clonazepam Clonopin (10) Clorazepate Tranxene (11) Clotiazepam Copyright ©2013 -

Was Marketed and Are Still Occumng. 168' 1699 172, 173, 175, 177-182, 185 Meprobamate Has Been Shown to Pro- 176
40 H. ISBELL & T. L. CHRUSCIEL CARBAMIC ACID ESTERS OF GLYCOLS Of the drugs listed in Table VI, carisoprodol 168. Bulla, J. D., Ewing, J. A. & Buffaloe, W. J. (1959) (S 103), emylcamate (S 104), mebutamate (S 107) Amer. Practit., 10, 1961 (Further controlled and phenprobamate (S 110) are not used primarily studies of meprobamate) as central nervous system depressants but are 169. Czerwenka-Wenkstetten, H., Hofmann, G. & recommended either for hypertension (S 107) or as Kryspin-Exner, K. (1965) Wien. med. Wschr., 115, 1012-1016 (Tranquillizersucht und Miss- " muscle relaxants " (S 103, S 104 and S 110). Some brauch) newly introduced drugs, buramate (S 102) and 170. Domino, E. F. (1965) In: DiPalma, J. R., ed., pentabamate (S 109), have been marketed as " tran- Drill's pharmacology in medicine, 3rd ed., quillizers" and hexapropymate (S 105) and oxy- pp. 356-364, McGraw-Hill, New York (Chap- fenamate (S 106) are used as sedatives. All these are ter 24: Psychosedative drugs. II. Meprobamnate, weak drugs and have not been abused or shown to chlordiazepoxide and miscellaneous agents) have abuse potential in animal or human experi- 171. Essig, C. F. (1958) Arch. Neurol. Psychiat. (Chic.), ments. 80, 414-417 (Withdrawal convulsions in dog Tybamate (S 111) used as a sedative has had an following chronic meprobamate intoxication) especially good animal and experimental work-up 172. Essig, C. F. & Ainslie, J. D. (1957) J. Amer. med. for abuse potential. 167, 170, 174, 187 It appears to be a Ass., 164, 1382 (Addiction to meprobamate) very weak, short-acting drug of no or very low 173. -

21 CFR Ch. II (4–1–01 Edition) § 1308.11
§ 1308.11 21 CFR Ch. II (4–1–01 Edition) and on certain order forms issued by (4) Alphacetylmethadol (except levo- the Administration pursuant to alphacetylmethadol also known as levo-alpha- acetylmethadol, levomethadyl acetate, or LAAM) ..... 9603 § 1305.05(d) of this chapter. Applicants (5) Alphameprodine ....................................................... 9604 for procurement and/or individual man- (6) Alphamethadol ......................................................... 9605 ufacturing quotas must include the ap- (7) Alpha-methylfentanyl (N-[1-(alpha-methyl-beta- propriate code number on the applica- phenyl)ethyl-4-piperidyl] propionanilide; 1-(1-methyl- 2-phenylethyl)-4-(N-propanilido) piperidine) ............... 9814 tion as required in §§ 1303.12(b) and (8) Alpha-methylthiofentanyl (N-[1-methyl-2-(2- 1303.22(a) of this chapter. Applicants thienyl)ethyl-4-piperidinyl]-N-phenylpropanamide) ..... 9832 for import and export permits must in- (9) Benzethidine ............................................................. 9606 (10) Betacetylmethadol .................................................. 9607 clude the appropriate code number on (11) Beta-hydroxyfentanyl (N-[1-(2-hydroxy-2- the application as required in phenethyl)-4-piperidinyl]-N-phenylpropanamide) ....... 9830 §§ 1312.12(a) and 1312.22(a) of this chap- (12) Beta-hydroxy-3-methylfentanyl (other name: N-[1- ter. Authorized registrants who desire (2-hydroxy-2-phenethyl)-3-methyl-4-piperidinyl]-N- phenylpropanamide .................................................... 9831 -

Michigan Burden Document Update
Michigan Burden Document Update Focusing on Abuse of Alcohol, Prescription Drugs, and Tobacco September 2010 Michigan Department of Community Health (MDCH) Bureau of Substance Abuse and Addiction Services (BSAAS) Strategic Planning Framework, State Incentive Grant (SPF/SIG) State Epidemiology Workgroup (SEW) Michigan Burden Document Update September 2010 INTRODUCTION Purpose This document serves as an update to the original “SPF/SIG MICHIGAN STATE EPIDEMIOLOGICAL WORKGROUP (SEW) SUMMARY TO THE STATE SIG ADVISORY COUNCIL (SAC), DESCRIBING THE BURDEN OF ALCOHOL, TOBACCO AND OTHER DRUGS ON THE STATE OF MICHIGAN,” December 2005. The State Epidemiology Workgroup (SEW) of the Strategic Planning Framework State Incentive Grant (SPF/SIG) produced both documents. Both the original and the updated versions describe the burden of alcohol, tobacco, and other drugs on the state of Michigan, however some differences are notable. The original version tallied the data available as of 2005, and served as a basis for the state to set priorities and to develop logic models for prevention efforts. This update is more narrowly focused on specific priorities set by the SEW in April of 2009, utilizing data available as of January 2010. In setting these priorities, the SEW considered readiness, political will, capacity, and resources in the ranking process. The priorities are noted in italics in the following table (Intro-Table 1). Primary users of this document are expected to be: federal partners, other state-level agencies, legislators, policy makers, elected officials, regional coordinating agencies and coalitions. For further information, contact the Michigan Department of Community Health, Bureau of Substance Abuse and Addiction Services, 320 S. -
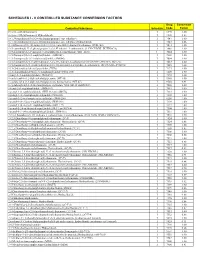
Controlled Substance Conversion Factors
SCHEDULES I - V CONTROLLED SUBSTANCE CONVERSION FACTORS Drug Conversion Controlled Substance Schedule Code Factor (+/-)cis -4-Methylaminorex I 1590 1.00 (+/-)cis -4-Methylaminorex Hydrochloride I 1590 0.83 1-(1,3-benzodioxol-5-yl)-2-(ethylamino)propan-1-one (ethylone) I 7547 1.00 1-(1,3-benzodioxol-5-yl)-2-(ethylamino)propan-1-one (ethylone) hydrochloride I 7547 0.86 (1-(4-fluorobenzyl)-1H -indol-3-yl)(2,2,3,3-tetramethylcyclopropyl)methanone (FUB-144) I 7014 1.00 1-(4-cyanobutyl)-N -(2-phenylpropan-2-yl)-1H -indazole-3-carboxamide (4-CN-CUMYL-BUTINACA) I 7089 1.00 1-(5-fluoropentyl)-1H -indazol-3-yl](naphthalen-1-yl)methanone (THJ–2201) 1 7024 1.00 1-(5-fluoropentyl)-3-(1-naphthoyl)indole (AM2201) I 7201 1.00 1-(5-fluoropentyl)-3-(2-iodobenzoyl)indole (AM694) I 7694 1.00 1-(5-fluoropentyl)-N -(2-phenylpropan-2-yl)-1H -indazole-3-carboxamide (5F-CUMYL-PINACA; SGT-25) I 7083 1.00 1-(5-fluoropentyl)-N -(2-phenylpropan-2-yl)-1H -pyrrolo[2,3-b]pyridine-3-carboxamide (5F-CUMYL-P7AICA) I 7085 1.00 1-[1-(2-thienyl)cyclohexyl]pyrrolidine (TCPy) I 7473 1.00 1-[2-(4-morpholinyl)ethyl]-3-(1-naphthoyl)indole (JWH- 200) I 7200 1.00 1-butyl-3-(1-naphthoyl)indole (JWH-073) I 7173 1.00 1-cyclohexyl-4-(1,2-diphenylethyl)piperazine (MT-45) I 9560 1.00 1-cyclohexyl-4-(1,2-diphenylethyl)piperazine hydrochloride (MT-45) I 9560 0.91 1-cyclohexylethyl-3-(2-methoxyphenylacetyl)indole 7008 (SR-18 and RCS-8) I 7008 1.00 1-hexyl-3-(1-naphthoyl)indole (JWH-019) I 7019 1.00 1-pentyl-3-(1-naphthoyl)indole (JWH-018 and AM678) I 7118 1.00 1-pentyl-3-(2-chlorophenylacetyl)indole -

SENATE BILL No. 298
Session of 2021 SENATE BILL No. 298 By Committee on Federal and State Affairs 3-16 1 AN ACT concerning the uniform controlled substances act; relating to 2 substances included in schedules I, II, IV and V; amending K.S.A. 65- 3 4107, 65-4111 and 65-4113 and K.S.A. 2020 Supp. 65-4105 and 4 repealing the existing sections. 5 6 Be it enacted by the Legislature of the State of Kansas: 7 Section 1. K.S.A. 2020 Supp. 65-4105 is hereby amended to read as 8 follows: 65-4105. (a) The controlled substances listed in this section are 9 included in schedule I and the number set forth opposite each drug or 10 substance is the DEA controlled substances code that has been assigned to 11 it. 12 (b) Any of the following opiates, including their isomers, esters, 13 ethers, salts, and salts of isomers, esters and ethers, unless specifically 14 excepted, whenever the existence of these isomers, esters, ethers and salts 15 is possible within the specific chemical designation: 16 (1) Acetyl fentanyl (N-(1-phenethylpiperidin-4-yl)-N- 17 phenylacetamide)..........................................................................9821 18 (2) Acetyl-alpha-methylfentanyl (N-[1-(1-methyl-2-phenethyl)-4- 19 piperidinyl]-N-phenylacetamide)..................................................9815 20 (3) Acetylmethadol.............................................................................9601 21 (4) Acryl fentanyl (N-(1-phenethylpiperidin-4-yl)-N-phenylacrylamide; 22 acryloylfentanyl)...........................................................................9811 23