Neurosteroids: Pregnenolone in Human Sciatic Nerves
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Exploring the Activity of an Inhibitory Neurosteroid at GABAA Receptors
1 Exploring the activity of an inhibitory neurosteroid at GABAA receptors Sandra Seljeset A thesis submitted to University College London for the Degree of Doctor of Philosophy November 2016 Department of Neuroscience, Physiology and Pharmacology University College London Gower Street WC1E 6BT 2 Declaration I, Sandra Seljeset, confirm that the work presented in this thesis is my own. Where information has been derived from other sources, I can confirm that this has been indicated in the thesis. 3 Abstract The GABAA receptor is the main mediator of inhibitory neurotransmission in the central nervous system. Its activity is regulated by various endogenous molecules that act either by directly modulating the receptor or by affecting the presynaptic release of GABA. Neurosteroids are an important class of endogenous modulators, and can either potentiate or inhibit GABAA receptor function. Whereas the binding site and physiological roles of the potentiating neurosteroids are well characterised, less is known about the role of inhibitory neurosteroids in modulating GABAA receptors. Using hippocampal cultures and recombinant GABAA receptors expressed in HEK cells, the binding and functional profile of the inhibitory neurosteroid pregnenolone sulphate (PS) were studied using whole-cell patch-clamp recordings. In HEK cells, PS inhibited steady-state GABA currents more than peak currents. Receptor subtype selectivity was minimal, except that the ρ1 receptor was largely insensitive. PS showed state-dependence but little voltage-sensitivity and did not compete with the open-channel blocker picrotoxinin for binding, suggesting that the channel pore is an unlikely binding site. By using ρ1-α1/β2/γ2L receptor chimeras and point mutations, the binding site for PS was probed. -

Pregnenolone Introduced 1997
Product Information Sheet – September 2016 Pregnenolone Introduced 1997 What Is It? • This product should not be taken by individuals with healthy levels of pregnenolone. Pregnenolone, 3-alpha-hydroxy-5-beta-pregnen-20-one, is a precursor to over 150 steroid hormones and is produced naturally in • Pregnenolone is best utilized by individuals over 40, and should the body from cholesterol. not be used to enhance athletic ability or endurance. Uses For Pregnenolone Are There Any Precautions Or Potential Side Memory Support: Animal studies have reported that pregnenolone Effects? may help to enhance memory by modulating N-methyl-D-aspartate Precautions: (NMDA) and gamma aminobutyrate (GABA) receptor activity in the brain. One study suggested that pregnenolone helped promote • NOT FOR USE BY INDIVIDUALS UNDER THE AGE OF 18. post-training task learning and memory.* • DO NOT USE IF PREGNANT OR NURSING. Immune Health: One study indicated that the 7-hydroxy metabolites • KEEP OUT OF REACH OF CHILDREN. from pregnenolone may help promote healthy immune system • Consult a physician or licensed qualified health professional before response.* using this product if you have, or have a family history of breast Mood Support: Pregnenolone has been reported to help promote cancer, prostate cancer, prostate enlargement, heart disease, low feelings of emotional well-being. One study suggested that “good” cholesterol (HDL), or if you are using any other dietary pregnenolone supported positive mood and feelings of motivation supplement, prescription drug, or over-the-counter drug. by mediating dopamine release.* • Do not exceed the recommended serving. Exceeding the What Is The Source? recommended serving may cause serious adverse health effects. -

RAC-GWVI: Research Alerts—Pubmed Citations for August 21 to 28, 2018 1
RAC-GWVI: Research Alerts—PubMed Citations for August 21 to 28, 2018 1 GULF WAR ILLNESS Neurotoxicity in acute and repeated organophosphate exposure. Naughton SX1, Terry AV Jr2. Toxicology. 2018 Aug 22. pii: S0300-483X(18)30264-6. doi: 10.1016/j.tox.2018.08.011. PMID: 30144465. [Epub ahead of print] The term organophosphate (OP) refers to a diverse group of chemicals that are found in hundreds of products worldwide. As pesticides, their most common use, OPs are clearly beneficial for agricultural productivity and the control of deadly vector-borne illnesses. However, as a consequence of their widespread use, OPs are now among the most common synthetic chemicals detected in the environment as well as in animal and human tissues. This is an increasing environmental concern because many OPs are highly toxic and both accidental and intentional exposures to OPs resulting in deleterious health effects have been documented for decades. Some of these deleterious health effects include a variety of long-term neurological and psychiatric disturbances including impairments in attention, memory, and other domains of cognition. Moreover, some chronic illnesses that manifest these symptoms such as Gulf War Illness and Aerotoxic Syndrome have (at least in part) been attributed to OP exposure. In addition to acute acetylcholinesterase inhibition, OPs may affect a number of additional targets that lead to oxidative stress, axonal transport deficits, neuroinflammation, and autoimmunity. Some of these targets could be exploited for therapeutic purposes. The purpose of this review is thus to: 1) describe the important uses of organophosphate (OP)-based compounds worldwide, 2) provide an overview of the various risks and toxicology associated with OP exposure, particularly long-term neurologic and psychiatric symptoms, 3) discuss mechanisms of OP toxicity beyond cholinesterase inhibition, 4) review potential therapeutic strategies to reverse the acute toxicity and long term deleterious effects of OPs. -

Calcium-Engaged Mechanisms of Nongenomic Action of Neurosteroids
Calcium-engaged Mechanisms of Nongenomic Action of Neurosteroids The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters Citation Rebas, Elzbieta, Tomasz Radzik, Tomasz Boczek, and Ludmila Zylinska. 2017. “Calcium-engaged Mechanisms of Nongenomic Action of Neurosteroids.” Current Neuropharmacology 15 (8): 1174-1191. doi:10.2174/1570159X15666170329091935. http:// dx.doi.org/10.2174/1570159X15666170329091935. Published Version doi:10.2174/1570159X15666170329091935 Citable link http://nrs.harvard.edu/urn-3:HUL.InstRepos:37160234 Terms of Use This article was downloaded from Harvard University’s DASH repository, and is made available under the terms and conditions applicable to Other Posted Material, as set forth at http:// nrs.harvard.edu/urn-3:HUL.InstRepos:dash.current.terms-of- use#LAA 1174 Send Orders for Reprints to [email protected] Current Neuropharmacology, 2017, 15, 1174-1191 REVIEW ARTICLE ISSN: 1570-159X eISSN: 1875-6190 Impact Factor: 3.365 Calcium-engaged Mechanisms of Nongenomic Action of Neurosteroids BENTHAM SCIENCE Elzbieta Rebas1, Tomasz Radzik1, Tomasz Boczek1,2 and Ludmila Zylinska1,* 1Department of Molecular Neurochemistry, Faculty of Health Sciences, Medical University of Lodz, Poland; 2Boston Children’s Hospital and Harvard Medical School, Boston, USA Abstract: Background: Neurosteroids form the unique group because of their dual mechanism of action. Classically, they bind to specific intracellular and/or nuclear receptors, and next modify genes transcription. Another mode of action is linked with the rapid effects induced at the plasma membrane level within seconds or milliseconds. The key molecules in neurotransmission are calcium ions, thereby we focus on the recent advances in understanding of complex signaling crosstalk between action of neurosteroids and calcium-engaged events. -

DHEA and the Brain Nutrition, Brain and Behaviour
DHEA and the Brain Nutrition, brain and behaviour Edited by Chandan Prasad, PhD Professor and Vice Chairman (Research) Department of Medicine LSU Health Sciences Center New Orleans, LA, USA Series Editorial Advisory Board Janina R.Galler, MD Director and Professor of Psychiatry and Public Health Center for Behavioral Development and Mental Retardation Boston University School of Medicine Boston, MA, USA R.C.A.Guedes, MD, Phd Departmento de Nutrição Centro de Ciências Da Saúde Universidade Federal de Pernambuco Recife/PE, BRASIL Gerald Huether, PhD Department of Psychiatric Medicine Georg-August-Universität Göttingen D-37075 Göttingen, Germany Abba J.Kastin, MD, DSc Editor-in-chief, PEPTIDES Endocrinology Section, Medical Service, V.A. Medical Center, 1601 Perdido Street, New Orleans, LA, USA H.R.Lieberman, PhD Military Performance and Neuroscience Division, USARIEM Natick, MA, USA DHEA and the Brain Edited by Robert Morfin Laboratoire de Biotechnologie Conservatoire National des Arts et Métiers Paris, France London and New York First published 2002 by Taylor & Francis 11 New Fetter Lane, London EC4P 4EE Simultaneously published in the USA and Canada by Taylor & Francis Inc, 29 West 35th Street, New York, NY 10001 Taylor & Francis is an imprint of the Toylor & Francis Group This edition published in the Taylor & Francis e-Library, 2005. “To purchase your own copy of this or any of Taylor & Francis or Routledge's collection of thousands of eBooks please go to www.eBookstore.tandf.co.uk.” © 2002 Taylor & Francis All rights reserved. No part of this book may be reprinted or reproduced or utilized in any form or by any electronic, mechanical, or other means, now known or hereafter invented, including photocopying and recording, or in any information storage or retrieval system, without permission in writing from the publishers. -

Comparison of Pregnenolone Sulfate, Pregnanolone and Estradiol Levels
Rustichelli et al. The Journal of Headache and Pain (2021) 22:13 The Journal of Headache https://doi.org/10.1186/s10194-021-01231-9 and Pain SHORT REPORT Open Access Comparison of pregnenolone sulfate, pregnanolone and estradiol levels between patients with menstrually-related migraine and controls: an exploratory study Cecilia Rustichelli1, Elisa Bellei2, Stefania Bergamini2, Emanuela Monari2, Flavia Lo Castro3, Carlo Baraldi4, Aldo Tomasi2 and Anna Ferrari4* Abstract Background: Neurosteroids affect the balance between neuroexcitation and neuroinhibition but have been little studied in migraine. We compared the serum levels of pregnenolone sulfate, pregnanolone and estradiol in women with menstrually-related migraine and controls and analysed if a correlation existed between the levels of the three hormones and history of migraine and age. Methods: Thirty women (mean age ± SD: 33.5 ± 7.1) with menstrually-related migraine (MM group) and 30 aged- matched controls (mean age ± SD: 30.9 ± 7.9) participated in the exploratory study. Pregnenolone sulfate and pregnanolone serum levels were analysed by liquid chromatography-tandem mass spectrometry, while estradiol levels by enzyme-linked immunosorbent assay. Results: Serum levels of pregnenolone sulfate and pregnanolone were significantly lower in the MM group than in controls (pregnenolone sulfate: P = 0.0328; pregnanolone: P = 0.0271, Student’s t-test), while estradiol levels were similar. In MM group, pregnenolone sulfate serum levels were negatively correlated with history of migraine (R2 = 0.1369; P = 0.0482) and age (R2 = 0.2826, P = 0.0025) while pregnenolone sulfate levels were not age-related in the control group (R2 = 0.04436, P = 0.4337, linear regression analysis). -

Bulk Drug Substances Nominated for Use in Compounding Under Section 503B of the Federal Food, Drug, and Cosmetic Act
Updated June 07, 2021 Bulk Drug Substances Nominated for Use in Compounding Under Section 503B of the Federal Food, Drug, and Cosmetic Act Three categories of bulk drug substances: • Category 1: Bulk Drug Substances Under Evaluation • Category 2: Bulk Drug Substances that Raise Significant Safety Risks • Category 3: Bulk Drug Substances Nominated Without Adequate Support Updates to Categories of Substances Nominated for the 503B Bulk Drug Substances List1 • Add the following entry to category 2 due to serious safety concerns of mutagenicity, cytotoxicity, and possible carcinogenicity when quinacrine hydrochloride is used for intrauterine administration for non- surgical female sterilization: 2,3 o Quinacrine Hydrochloride for intrauterine administration • Revision to category 1 for clarity: o Modify the entry for “Quinacrine Hydrochloride” to “Quinacrine Hydrochloride (except for intrauterine administration).” • Revision to category 1 to correct a substance name error: o Correct the error in the substance name “DHEA (dehydroepiandosterone)” to “DHEA (dehydroepiandrosterone).” 1 For the purposes of the substance names in the categories, hydrated forms of the substance are included in the scope of the substance name. 2 Quinacrine HCl was previously reviewed in 2016 as part of FDA’s consideration of this bulk drug substance for inclusion on the 503A Bulks List. As part of this review, the Division of Bone, Reproductive and Urologic Products (DBRUP), now the Division of Urology, Obstetrics and Gynecology (DUOG), evaluated the nomination of quinacrine for intrauterine administration for non-surgical female sterilization and recommended that quinacrine should not be included on the 503A Bulks List for this use. This recommendation was based on the lack of information on efficacy comparable to other available methods of female sterilization and serious safety concerns of mutagenicity, cytotoxicity and possible carcinogenicity in use of quinacrine for this indication and route of administration. -

Research Article
Available Online at http://www.recentscientific.com International Journal of CODEN: IJRSFP (USA) Recent Scientific International Journal of Recent Scientific Research Research Vol. 9, Issue, 6(E), pp. 27560-27565, June, 2018 ISSN: 0976-3031 DOI: 10.24327/IJRSR Research Article BIOSYNTHESIS OF NEUROSTEROID AND PHARMACOLOGYCAL ACTION Vandna Dewangan*., Trilochan Satapthy and Ram Sahu Department of Pharmacology, Columbia Institute of Pharmacy, Tekari, Near Vidhansabha, Raipur-493111(C.G.) India DOI: http://dx.doi.org/10.24327/ijrsr.2018.0906.2285 ARTICLE INFO ABSTRACT Article History: Over the past decade, it has become clear that the brain, like the gonad, adrenal and placenta, is a steroid genic organ. Neurosteroids are synthetized in the central and the peripheral nervous system, Received 20th March, 2018 in glial cells, and also in neurons, from cholesterol or steroidal precursors imported from peripheral Received in revised form 27th sources. However, unlike classic steroid genic tissues, the synthesis of steroids in the nervous April, 2018 system requires the coordinate expression and regulation of the genes encoding the steroid genic Accepted 5th May, 2018 enzymes in several different cell types (neurons and glia) at different locations in the nervous Published online 28th June, 2018 system, and at distances from the cell bodies. The steroids synthesized by the brain and nervous system, given the name neurosteroids Progesterone itself is also a neurosteroid, and a progesterone Key Words: receptor has been detected in peripheral and central glial cells. At different sites in the brain, Neurosteroid, Steroid hormones, neurosteroid concentrations vary according to environmental and behavioural circumstances, such as Progesterone, Glial Cell, Nuclear receptor, stress, sex recognition, or aggressiveness. -

Pregnenolone, a Fruit of Cholesterol
PREGNENOLONE, A FRUIT OF CHOLESTEROL Mother of Progesterone & D.H.E.A. The following information comes from Dr. Ray Peat, who has done pioneering research on the anti-aging steroids, pregnenolone, progesterone and DHEA (Dehydroepiandrosterone). I have included excerpts from his writings plus the results of interviews. Research references are provided when available, but in many cases, I could only describe the group of researchers who did the experiment. My purpose for this article is not to start a riot but to illustrate why I think it’s dangerous to artificially inhibit cholesterol formation in your body with drugs and synthetic foods. Dr. Peat accidentally discovered the effects of pregnenolone when he took some vitamin E containing a residue of pregnenolone that was left over from an experiment in solubility. Peat had been suffering from a variety of complaints, including " inflammation of the arteries, dental abscesses, asthma, migraines, and colitis ." When he took the vitamin E containing some pregnenolone in the vitamin E, he crawled out of his sick bed, took a pinch of pure pregnenolone and felt immediately better. All of his symptoms gradually disappeared and in ten weeks, his appearance changed. Many aging characteristics, such as sagging skin, "chicken neck," bags under the eyes, etc. receded. These changes were dramatically reported in a passport photo, taken one year before pregnenolone and another one taken 10 weeks after pregnenolone therapy was initiated. I regret that the original photos are not available for inclusion here. When I saw this photo, presented in one of his newsletters, I fell off my chair, dashed to the phone and called Dr. -
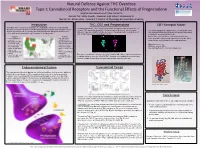
Introduction Endocannabinoid System Cannabinoid Tetrad THC, CB1 And
Natural Defense Against THC Overdose Type-1 Cannabinoid Receptors and the Functional Effects of Pregnenolone Brigitte Rios Llamosa and Alexis Camacho Advisor: Mr. Justin Spaeth, Messmer High School, Milwaukee WI Mentor: Dr. Aaron Miller - Assistant Professor of Physiology at Concordia University Introduction THC, CB1 and Pregnenolone CB1 Receptor Model Cannabis, often referred to as marijuana, is a drug produced from the Cannabis plant. Tetrahydrocannabinol (THC), the active ingredient in marijuana, also activates the CB1 Recently, marijuana has become an often debated topic as people work to legalize its use receptor. THC and similar drugs have therapeutic potential in the treatment of pain, Our model highlights the amino acids E133 and R409, which for both recreational (as in Colorado) and medical purposes. Marijuana is able to help Alzheimer’s disease, anxiety, arthritis, and cancer. A downside to the medicinal use of form hydrogen bonds with pregnenolone and are required for relieve pain, but it can also lower performance in everyday tasks. THC is that it also induces psychotropic effects. its binding to the allosteric site of CB1. Negatives Positives Both of these amino acids are colored in cpk color and • Poor coordination of • Can help control connected with a strut to our pregnenolone molecule. Other movement epileptic seizures areas that are highlighted are color-coded as follows: • Afterwards, users feel • May decrease anxiety tired or depressed • Can slow progression Alpha helixes are red. • Increases heartbeat and of diseases such as risk of heart attack Alzheimer’s disease Struts are colored white. • Inability to understand • Has been used to treat Non-motif portions are colored cornflower blue. -

Sigma, PCP, and NMDA Receptors
National Institute on Drug Abuse RESEARCH MONOGRAPH SERIES Sigma, PCP, and NMDA Receptors 133 U.S. Department of Health and Human Services • Public Health Service • National Institutes of Health Sigma, PCP, and NMDA Receptors Editors: Errol B. De Souza, Ph.D. Addiction Research Center National Institute on Drug Abuse Doris Clouet, Ph.D. Division of Basic Research National Institute on Drug Abuse Edythe D. London, Ph.D. Addiction Research Center National Institute on Drug Abuse Research Monograph 133 1993 U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES Public Health Service Substance Abuse and Mental Health Services Administration National Institute on Drug Abuse 5600 Fishers Lane Rockville, MD 20857 ACKNOWLEDGMENT This monograph is based on the papers and discussions from a technical review on “Sigma, PCP, and NMDA Receptors Systems” held on September 27-28, 1989, in Baltimore, MD. The review meeting was sponsored by the National Institute on Drug Abuse. COPYRIGHT STATUS The National Institute on Drug Abuse has obtained permission from the copyright holders to reproduce certain previously published material as noted in the text. Further reproduction of this copyrighted material is permitted only as part of a reprinting of the entire publication or chapter. For any other use, the copyright holder’s permission is required. All other material in this volume except quoted passages from copyrighted sources is in the public domain and may be used or reproduced without permission from the Institute or the authors. Citation of the source is appreciated. Opinions expressed in this volume are those of the authors and do not necessarily reflect the opinions or official policy of the National Institute on Drug Abuse or any other part of the Department of Health and Human Services. -

Michigan Medicaid Program Maximum Allowable Cost (MAC) Pricing
1 ** Confidential and Proprietary ** For Week Ending 09/22/2021 Michigan Medicaid Program Maximum Allowable Cost (MAC) Pricing Due to frequent changes in price and product availability, this listing should NOT be considered all-inclusive. Updated listings will be posted weekly. Effective MAC Generic Name Strength Form Route Date Price 0.9 % SODIUM CHLORIDE 0.9 % VIAL INJECTION 01/27/2021 0.07271 0.9 % SODIUM CHLORIDE 0.9 % IV SOLN INTRAVEN 03/17/2021 0.00327 1,2-PENTANEDIOL 100 % LIQUID MISCELL 10/28/2020 0.56682 2-DEOXY-D-GLUCOSE POWDER MISCELL 12/09/2020 54.63250 5-HYDROXYTRYPTOPHAN (5-HTP) 100 MG CAPSULE ORAL 01/20/2021 0.33500 5HYDROXYTRYPTOPHAN(OXITRIPTAN) POWDER MISCELL 12/16/2020 11.24640 7-OXODEHYDROEPIANDROSTERONE,MC 100 % POWDER MISCELL 10/07/2020 7.36092 ABACAVIR SULFATE 20 MG/ML SOLUTION ORAL 12/30/2020 0.67190 ABACAVIR SULFATE 300 MG TABLET ORAL 06/09/2021 0.89065 ABACAVIR SULFATE/LAMIVUDINE 600-300MG TABLET ORAL 03/10/2021 2.11005 ABACAVIR/LAMIVUDINE/ZIDOVUDINE 150-300 MG TABLET ORAL 12/11/2019 26.74276 ABIRATERONE ACETATE 500 MG TABLET ORAL 09/20/2021 145.40750 ACACIA POWDER MISCELL 01/22/2020 0.14149 ACAI BERRY EXTRACT 500 MG CAPSULE ORAL 05/01/2019 0.06689 ACAMPROSATE CALCIUM 333 MG TABLET DR ORAL 07/29/2020 0.97128 ACARBOSE 100 MG TABLET ORAL 09/08/2021 0.40079 ACARBOSE 50 MG TABLET ORAL 11/11/2020 0.28140 ACARBOSE 25 MG TABLET ORAL 01/13/2021 0.22780 ACEBUTOLOL HCL 200 MG CAPSULE ORAL 09/08/2021 0.88574 ACEBUTOLOL HCL 400 MG CAPSULE ORAL 03/31/2021 1.02443 ACESULFAME POTASSIUM 100 % POWDER MISCELL 12/09/2020 3.34620 MI MAC Pricing Information contained in this document is confidential and proprietary and is available to you solely for the purpose of assisting with claim processing and program reimbursement analysis.