ERG Dependence Distinguishes Developmental Control of Hematopoietic Stem Cell Maintenance from Hematopoietic Specification
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ubiquitin-Mediated Control of ETS Transcription Factors: Roles in Cancer and Development
International Journal of Molecular Sciences Review Ubiquitin-Mediated Control of ETS Transcription Factors: Roles in Cancer and Development Charles Ducker * and Peter E. Shaw * Queen’s Medical Centre, School of Life Sciences, University of Nottingham, Nottingham NG7 2UH, UK * Correspondence: [email protected] (C.D.); [email protected] (P.E.S.) Abstract: Genome expansion, whole genome and gene duplication events during metazoan evolution produced an extensive family of ETS genes whose members express transcription factors with a conserved winged helix-turn-helix DNA-binding domain. Unravelling their biological roles has proved challenging with functional redundancy manifest in overlapping expression patterns, a common consensus DNA-binding motif and responsiveness to mitogen-activated protein kinase signalling. Key determinants of the cellular repertoire of ETS proteins are their stability and turnover, controlled largely by the actions of selective E3 ubiquitin ligases and deubiquitinases. Here we discuss the known relationships between ETS proteins and enzymes that determine their ubiquitin status, their integration with other developmental signal transduction pathways and how suppression of ETS protein ubiquitination contributes to the malignant cell phenotype in multiple cancers. Keywords: E3 ligase complex; deubiquitinase; gene fusions; mitogens; phosphorylation; DNA damage 1. Introduction Citation: Ducker, C.; Shaw, P.E. Cell growth, proliferation and differentiation are complex, concerted processes that Ubiquitin-Mediated Control of ETS Transcription Factors: Roles in Cancer rely on careful regulation of gene expression. Control over gene expression is maintained and Development. Int. J. Mol. Sci. through signalling pathways that respond to external cellular stimuli, such as growth 2021, 22, 5119. https://doi.org/ factors, cytokines and chemokines, that invoke expression profiles commensurate with 10.3390/ijms22105119 diverse cellular outcomes. -

Inducible Transgene Expression in PDX Models
Liu et al. Biomarker Research (2020) 8:46 https://doi.org/10.1186/s40364-020-00226-z RESEARCH Open Access Inducible transgene expression in PDX models in vivo identifies KLF4 as a therapeutic target for B-ALL Wen-Hsin Liu1, Paulina Mrozek-Gorska2, Anna-Katharina Wirth1, Tobias Herold1,3, Larissa Schwarzkopf1, Dagmar Pich2, Kerstin Völse1, M. Camila Melo-Narváez2, Michela Carlet1, Wolfgang Hammerschmidt2,4 and Irmela Jeremias1,5,6* Abstract Background: Clinically relevant methods are not available that prioritize and validate potential therapeutic targets for individual tumors, from the vast amount of tumor descriptive expression data. Methods: We established inducible transgene expression in clinically relevant patient-derived xenograft (PDX) models in vivo to fill this gap. Results: With this technique at hand, we analyzed the role of the transcription factor Krüppel-like factor 4 (KLF4) in B-cell acute lymphoblastic leukemia (B-ALL) PDX models at different disease stages. In competitive preclinical in vivo trials, we found that re-expression of wild type KLF4 reduced the leukemia load in PDX models of B-ALL, with the strongest effects being observed after conventional chemotherapy in minimal residual disease (MRD). A nonfunctional KLF4 mutant had no effect on this model. The re-expression of KLF4 sensitized tumor cells in the PDX model towards systemic chemotherapy in vivo. It is of major translational relevance that azacitidine upregulated KLF4 levels in the PDX model and a KLF4 knockout reduced azacitidine-induced cell death, suggesting that azacitidine can regulate KLF4 re-expression. These results support the application of azacitidine in patients with B-ALL as a therapeutic option to regulate KLF4. -

Ten Commandments for a Good Scientist
Unravelling the mechanism of differential biological responses induced by food-borne xeno- and phyto-estrogenic compounds Ana María Sotoca Covaleda Wageningen 2010 Thesis committee Thesis supervisors Prof. dr. ir. Ivonne M.C.M. Rietjens Professor of Toxicology Wageningen University Prof. dr. Albertinka J. Murk Personal chair at the sub-department of Toxicology Wageningen University Thesis co-supervisor Dr. ir. Jacques J.M. Vervoort Associate professor at the Laboratory of Biochemistry Wageningen University Other members Prof. dr. Michael R. Muller, Wageningen University Prof. dr. ir. Huub F.J. Savelkoul, Wageningen University Prof. dr. Everardus J. van Zoelen, Radboud University Nijmegen Dr. ir. Toine F.H. Bovee, RIKILT, Wageningen This research was conducted under the auspices of the Graduate School VLAG Unravelling the mechanism of differential biological responses induced by food-borne xeno- and phyto-estrogenic compounds Ana María Sotoca Covaleda Thesis submitted in fulfillment of the requirements for the degree of doctor at Wageningen University by the authority of the Rector Magnificus Prof. dr. M.J. Kropff, in the presence of the Thesis Committee appointed by the Academic Board to be defended in public on Tuesday 14 September 2010 at 4 p.m. in the Aula Unravelling the mechanism of differential biological responses induced by food-borne xeno- and phyto-estrogenic compounds. Ana María Sotoca Covaleda Thesis Wageningen University, Wageningen, The Netherlands, 2010, With references, and with summary in Dutch. ISBN: 978-90-8585-707-5 “Caminante no hay camino, se hace camino al andar. Al andar se hace camino, y al volver la vista atrás se ve la senda que nunca se ha de volver a pisar” - Antonio Machado – A mi madre. -

RUNX1 Together with a Compendium of Hematopoietic Regulators, Chromatin Modifiers and Basal Transcr
Leukemia (2014) 28, 770–778 OPEN & 2014 Macmillan Publishers Limited All rights reserved 0887-6924/14 www.nature.com/leu ORIGINAL ARTICLE CBFB–MYH11/RUNX1 together with a compendium of hematopoietic regulators, chromatin modifiers and basal transcription factors occupies self-renewal genes in inv(16) acute myeloid leukemia A Mandoli1, AA Singh1, PWTC Jansen2, ATJ Wierenga3,4, H Riahi1, G Franci5, K Prange1, S Saeed1, E Vellenga3, M Vermeulen2, HG Stunnenberg1 and JHA Martens1 Different mechanisms for CBFb–MYH11 function in acute myeloid leukemia with inv(16) have been proposed such as tethering of RUNX1 outside the nucleus, interference with transcription factor complex assembly and recruitment of histone deacetylases, all resulting in transcriptional repression of RUNX1 target genes. Here, through genome-wide CBFb–MYH11-binding site analysis and quantitative interaction proteomics, we found that CBFb–MYH11 localizes to RUNX1 occupied promoters, where it interacts with TAL1, FLI1 and TBP-associated factors (TAFs) in the context of the hematopoietic transcription factors ERG, GATA2 and PU.1/SPI1 and the coregulators EP300 and HDAC1. Transcriptional analysis revealed that upon fusion protein knockdown, a small subset of the CBFb–MYH11 target genes show increased expression, confirming a role in transcriptional repression. However, the majority of CBFb–MYH11 target genes, including genes implicated in hematopoietic stem cell self-renewal such as ID1, LMO1 and JAG1, are actively transcribed and repressed upon fusion protein knockdown. Together these results suggest an essential role for CBFb–MYH11 in regulating the expression of genes involved in maintaining a stem cell phenotype. Leukemia (2014) 28, 770–778; doi:10.1038/leu.2013.257 Keywords: CBFb–MYH11; RUNX1; histone acetylation; acute myeloid leukemia; inv(16) INTRODUCTION Heterozygous Cbfb-Myh11 knock-in mice are embryonic lethal, Core-binding transcription factors (CBFs) have roles in stem cell with definitive hematopoiesis blocked at the stem-cell level. -

The Histone Methyltransferase MMSET/WHSC1 Activates TWIST1 to Promote an Epithelial–Mesenchymal Transition and Invasive Properties of Prostate Cancer
Oncogene (2013) 32, 2882–2890 & 2013 Macmillan Publishers Limited All rights reserved 0950-9232/13 www.nature.com/onc ORIGINAL ARTICLE The histone methyltransferase MMSET/WHSC1 activates TWIST1 to promote an epithelial–mesenchymal transition and invasive properties of prostate cancer T Ezponda1, R Popovic1, MY Shah1, E Martinez-Garcia1, Y Zheng2, D-J Min1, C Will1, A Neri3, NL Kelleher1,2,JYu1 and JD Licht1 Epigenetic deregulation of gene expression has a role in the initiation and progression of prostate cancer (PCa). The histone methyltransferase MMSET/WHSC1 (Multiple Myeloma SET domain) is overexpressed in a number of metastatic tumors, but its mechanism of action has not been defined. In this work, we found that PCa cell lines expressed significantly higher levels of MMSET compared with immortalized, non-transformed prostate cells. Knockdown experiments showed that, in metastatic PCa cell lines, dimethylation of lysine 36 and trimethylation of lysine 27 on histone H3 (H3K36me2 and H3K27me3, respectively) depended on MMSET expression, whereas depletion of MMSET in benign prostatic cells did not affect chromatin modifications. Knockdown of MMSET in DU145 and PC-3 tumor cells decreased cell proliferation, colony formation in soft agar and strikingly diminished cell migration and invasion. Conversely, overexpression of MMSET in immortalized, non-transformed RWPE-1 cells promoted cell migration and invasion, accompanied by an epithelial–mesenchymal transition (EMT). Among a panel of EMT-promoting genes analyzed, TWIST1 expression was strongly activated in response to MMSET. Chromatin immunoprecipitation analysis demonstrated that MMSET binds to the TWIST1 locus and leads to an increase in H3K36me2, suggesting a direct role of MMSET in the regulation of this gene. -

GATA3-Controlled Nucleosome Eviction Drives MYC Enhancer Activity in T-Cell Development and Leukemia
Published OnlineFirst September 13, 2019; DOI: 10.1158/2159-8290.CD-19-0471 RESEARCH ARTICLE GATA3-Controlled Nucleosome Eviction Drives MYC Enhancer Activity in T-cell Development and Leukemia Laura Belver1, Alexander Y. Yang1, Robert Albero1, Daniel Herranz2,3, Francesco G. Brundu4, S. Aidan Quinn1, Pablo Pérez-Durán1, Silvia Álvarez1, Francesca Gianni1, Marissa Rashkovan1, Devya Gurung1, Pedro P. Rocha5, Ramya Raviram6,7, Clara Reglero1, Jose R. Cortés1, Anisha J. Cooke1, Agnieszka A. Wendorff1, Valentina Cordó8, Jules P. Meijerink8, Raúl Rabadan4,9, and Adolfo A. Ferrando1,4,10,11 ABSTRACT Long-range enhancers govern the temporal and spatial control of gene express ion; however, the mechanisms that regulate enhancer activity during normal and malig- nant development remain poorly understood. Here, we demonstrate a role for aberrant chromatin acces- sibility in the regulation of MYC expression in T-cell lymphoblastic leukemia (T-ALL). Central to this process, the NOTCH1-MYC enhancer (N-Me), a long-range T cell–specificMYC enhancer, shows dynamic changes in chromatin accessibility during T-cell specification and maturation and an aberrant high degree of chromatin accessibility in mouse and human T-ALL cells. Mechanistically, we demonstrate that GATA3- driven nucleosome eviction dynamically modulates N-Me enhancer activity and is strictly required for NOTCH1-induced T-ALL initiation and maintenance. These results directly implicate aberrant regulation of chromatin accessibility at oncogenic enhancers as a mechanism of leukemic transformation. SIGNIFICANCE: MYC is a major effector of NOTCH1 oncogenic programs in T-ALL. Here, we show a major role for GATA3-mediated enhancer nucleosome eviction as a driver of MYC expression and leuke- mic transformation. -

The Erg Gene: a Human Gene Related to the Ets Oncogene (Cloning/Sequencing/Expression/Oncogene Homology) E
Proc. Nati. Acad. Sci. USA Vol. 84, pp. 6131-6135, September 1987 Biochemistry The erg gene: A human gene related to the ets oncogene (cloning/sequencing/expression/oncogene homology) E. SHYAM P. REDDY*t, VEENA N. RAOI, AND TAKIS S. PAPAS: tLaboratory of Molecular Oncology and *Programme Resources, Inc., National Cancer Institute, Frederick, MD 21701 Communicated by Max Tishler, May 26, 1987 ABSTRACT We have isolated a cDNA clone representing the complete coding sequence of a human gene named erg, RP C P R P R C R related to the ets oncogene. Nucleotide sequence analysis of this X7 ,i cDNA (4.6 kilobases long) revealed that this gene encodes a (erg 1) ATG TAA AATAAA 363-residue protein whose predicted amino acid sequence showed a homology of -40% and -7O% to two domains corresponding to the 5' and 3' regions of v-ets oncogene, 200 bp respectively. A 3.2- to 3.6-kilobase and -5-kilobase transcript FIG. 1. Analysis of the coding region of the -5-kb human ergl of the erg gene, which differ in size from those ofthe previously mRNA performed on a single phage "4.6-kb cDNA spanning the described Hu-ets 1 and Hu-ets 2 genes, were observed in entire region. Hatched boxes represent 5' (left) and 3' (right) different cells. These results suggest that the erg gene is a homologous regions of v-ets oncogene. Initiation codon (ATG), member of the ets oncogene family. termination codon (TAA), and polyadenylylation signal (AATAAA) are also shown. Horizontal arrow represents an 8-bp sequence, a avian which is repeated twice as a direct repeat in another cDNA clone E26 is replication-defective acute leukemia virus, (erg2). -

BAALC Potentiates Oncogenic ERK Pathway Through Interactions with MEKK1 and KLF4
Leukemia (2015) 29, 2248–2256 © 2015 Macmillan Publishers Limited All rights reserved 0887-6924/15 www.nature.com/leu ORIGINAL ARTICLE BAALC potentiates oncogenic ERK pathway through interactions with MEKK1 and KLF4 K Morita1, Y Masamoto1, K Kataoka1, J Koya1, Y Kagoya1, H Yashiroda2, T Sato1,3, S Murata2 and M Kurokawa1,4 Although high brain and acute leukemia, cytoplasmic (BAALC) expression is a well-characterized poor prognostic factor in acute myeloid leukemia (AML), neither the exact mechanisms by which BAALC drives leukemogenesis and drug resistance nor therapeutic approaches against BAALC-high AML have been properly elucidated. In this study, we found that BAALC induced cell- cycle progression of leukemia cells by sustaining extracellular signal-regulated kinase (ERK) activity through an interaction with a scaffold protein MEK kinase-1 (MEKK1), which inhibits the interaction between ERK and MAP kinase phosphatase 3 (MKP3/DUSP6). BAALC conferred chemoresistance in AML cells by upregulating ATP-binding cassette proteins in an ERK-dependent manner, which can be therapeutically targeted by MEK inhibitor. We also demonstrated that BAALC blocks ERK-mediated monocytic differentiation of AML cells by trapping Krüppel-like factor 4 (KLF4) in the cytoplasm and inhibiting its function in the nucleus. Consequently, MEK inhibition therapy synergizes with KLF4 induction and is highly effective against BAALC-high AML cells both in vitro and in vivo. Our data provide a molecular basis for the role of BAALC in regulating proliferation and differentiation of AML cells and highlight the unique dual function of BAALC as an attractive therapeutic target against BAALC-high AML. Leukemia (2015) 29, 2248–2256; doi:10.1038/leu.2015.137 INTRODUCTION Previous reports have suggested a fundamental role of MEKK1 in 11,12 Acute myeloid leukemia (AML) is a clonal disorder characterized anti-apoptotic function in myeloid cells and leukemogenesis. -

Genome-Wide DNA Methylation Events in TMPRSS2–ERG Fusion-Negative Prostate Cancers Implicate an EZH2-Dependent Mechanism with Mir-26A Hypermethylation
Published OnlineFirst August 28, 2012; DOI: 10.1158/2159-8290.CD-12-0041 RESEARCH ARTICLE Genome-wide DNA Methylation Events in TMPRSS2–ERG Fusion-Negative Prostate Cancers Implicate an EZH2-Dependent Mechanism with miR-26a Hypermethylation Stefan T. Börno 1 , 3 , Axel Fischer 1 , Martin Kerick 1 , Maria Fälth 4 , 6 , Mark Laible 4 , Jan C. Brase 4 , 7 , Ruprecht Kuner 4 , Andreas Dahl 8 , Christina Grimm 1 , Behnam Sayanjali 1 , Melanie Isau 1 , 3 , Christina Röhr 1 , 3 , Andrea Wunderlich 1 , 3 , Bernd Timmermann 2 , Rainer Claus 5 , Christoph Plass5 , Markus Graefen 9 , Ronald Simon 10 , Francesca Demichelis 11 , 13 , Mark A. Rubin 12 , Guido Sauter 10 , Thorsten Schlomm 9 , Holger Sültmann 4 , Hans Lehrach 1 , and Michal R. Schweiger 1 Downloaded from cancerdiscovery.aacrjournals.org on October 2, 2021. © 2012 American Association for Cancer Research. Published OnlineFirst August 28, 2012; DOI: 10.1158/2159-8290.CD-12-0041 ABSTRACT Prostate cancer is the second most common cancer among men worldwide. Altera- tions in the DNA methylation pattern can be one of the leading causes for prostate cancer formation. This study is the fi rst high-throughput sequencing study investigating genome-wide DNA methylation patterns in a large cohort of 51 tumor and 53 benign prostate samples using meth- ylated DNA immunoprecipitation sequencing. Comparative analyses identifi ed more than 147,000 cancer-associated epigenetic alterations. In addition, global methylation patterns show signifi cant differences based on the TMPRSS2–ERG rearrangement status. We propose the hypermethylation of miR-26a as an alternative pathway of ERG rearrangement-independent EZH2 activation. The observed increase in differential methylation events in fusion–negative tumors can explain the tumorigenic proc- ess in the absence of genomic rearrangements. -

ERG Promotes Transcriptional Reprogramming of Prostatic Epithelial Cells Toward a Cancer Stem Cell Phenotype
ERG promotes transcriptional reprogramming of prostatic epithelial cells toward a cancer stem cell phenotype Manju Sharma, Yubin Guo, Robert Bell, and Michael E. Cox. Vancouver Prostate Centre. Vancouver British Columbia, Canada Abstract Genomic rearrangements that result in aberrant expression of the proto-oncogene member of the erythroblast transformation-specific family of transcription factors, ERG, occur in almost half of prostate cancers. The most frequent rearrangements are the result of an interstitial deletion that results in fusion of androgen-responsive elements of serine 2-erythroblast transformation-specific-related gene with variable amino terminal ERG open reading frame exons. These TMPRSS2-ERG fusions are the most common cancer-associated mutation. They occur early in prostate cancer initiation, are associated with high Gleason score, and aggressive disease. When amplified and/or expressed at high level, TMPRSS2-ERG is associated with poor prognosis. Mirroring its functions in normal chondrocyte and endothelial cell differentiation, ERG expression in prostatic epithelial cells drives aberrant activation of transcriptional programs promoting migration, invasion, and epithelial-mesenchymal transition. Additionally, TMPRSS2-ERG status appears to enhance resistance to abiraterone and taxane therapies. We hypothesized that ERG expression can promote transformation of prostatic epithelial cells by directing transcriptional reprogramming towards a cancer stem cell phenotype. Transcriptional analysis of ERG-expressing RWPE1 prostatic epithelial cells (ERG-RWPE1) demonstrated up-regulation transcription of a panel of stem cell-related genes. When cultured under non- adherent conditions, ERG-RWPE1 cells selectively demonstrated the ability of form serially passable spheroids and dramatically increased expression of stem cell markers, PROM1, c-KIT and CD33. When re- plated in adherent conditions, cells re-established the expression pattern of parental adherent cultures. -
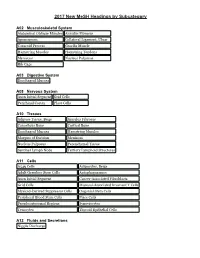
2017 New Mesh Headings by Subcategory
2017 New MeSH Headings by Subcategory A02 Musculoskeletal System Abdominal Oblique Muscles Annulus Fibrosus Aponeurosis Collateral Ligament, Ulnar Coracoid Process Gracilis Muscle Hamstring Muscles Hamstring Tendons Meniscus Nucleus Pulposus Rib Cage A03 Digestive System Esophageal Mucosa A08 Nervous System Axon Initial Segment Grid Cells Perirhinal Cortex Place Cells A10 Tissues Adipose Tissue, Beige Annulus Fibrosus Cancellous Bone Cortical Bone Esophageal Mucosa Hamstring Muscles Margins of Excision Meniscus Nucleus Pulposus Parenchymal Tissue Sentinel Lymph Node Tertiary Lymphoid Structures A11 Cells A549 Cells Adipocytes, Beige Adult Germline Stem Cells Autophagosomes Axon Initial Segment Cancer-Associated Fibroblasts Grid Cells Mucosal-Associated Invariant T Cells Myeloid-Derived Suppressor Cells Oogonial Stem Cells Peripheral Blood Stem Cells Place Cells Pseudoautosomal Regions Synoviocytes Tenocytes Thyroid Epithelial Cells A12 Fluids and Secretions Nipple Discharge 2017 New MeSH Headings by Subcategory A13 Animal Structures Gizzard, Non-avian A15 Hemic and Immune Systems Mucosal-Associated Invariant T Cells Sentinel Lymph Node Tertiary Lymphoid Structures A16 Embryonic Structures Gubernaculum B01 Eukaryota Amaryllidaceae Asparagaceae Asparagales Camelidae Colchicaceae Endamoeba histolytica Gentianales Hypoxidaceae Liliales Loteae Melanthiaceae Ocimum sanctum Pogostemon Psacalium Saccharomyces boulardii Vigna Wolfiporia Xanthorrhoeaceae 2017 New MeSH Headings by Subcategory B03 Bacteria Aeromonas veronii Bacillus amyloliquefaciens -

Engineered Stem Cells in Hematologic Diseases
Next Generation Transplantation: Engineered Stem Cells in Hematologic Diseases George Q. Daley Boston Children’s Hospital/ Harvard Medical School/ HHMI Tandem Meetings 2016 Disclosures: Scientific Advisory Boards (consulting fees and equity) MPM Capital, Solasia, Epizyme, Ocata, Raze, True North, Verastem, AthenaHealth Research Funding: Megakaryon Boston Children’s/ Dana Farber Pediatric Stem Cell Transplant 2015: 114 Transplants Leslie Lehmann Christy Duncan Nick Haining Clinical Director Assoc. Clinical Dir. Assoc. Scientific Dir. Alessandra Biffi Director Gene Therapy Steve Margossian Sung-Yun Pai Suneet Agarwal Michelle Lee Christy Ullirch Jen Whangbo Susanne Baumeister Esther Obang Leo Wang Birgit Knoechel Inga Hoffman Autologous Cell Therapy from iPS Cells NSG Transplant Skin or Blood Cell Primary Immune Deficiency Hematopoietic Bone Marrow Failure stem cells Hemoglobinopathy Reprogramming (Oct4/Sox2/Klf4/Myc) ? Gene Repair Diseased iPS cells Healthy iPS cells ES/ iPS Mammalian Embryoidblood development:Bodies Primitive and Definitive= Yolk Sac waves Morphogens? Drugs? Transcription Factors? Strategies for deriving HSCs from HUMAN pluripotent stem cells (ESC/iPSCs) HSC-specific TFs ?? Respecification CD34+43- ESC/iPSC Hemogenic HSC? CD34+ Endothelium CD45+ TFs Myeloid CFUs Specification Morphogens Respecifying blood progenitors from iPSCs hESC/iPSC EB differentiation - BMP4/cytokines CD34+45+ HPCs D0 D4-7 D11+ D14 HSC TFs 7+ days 9 HSC TFs: Transplant HLF ERG Control MYB SOX4 CD34+45+ KLF4 HPCs RORA Microarray HOXA5 HOXA9 HOXA10