Effect of Rhepo Administration on Serum Levels of Stfr and Cycling Performance
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Jukolan Viestin Tarina 1989-2006
JUKOLAN VIESTIEN TARINA II Vuodet 1989- Kaukametsäläiset ry 2006 Jukolan viestin tarina II Vuodet 1989- 2 Jukolan viestin tarina II Vuodet 1989- 3 ALKUSANAT Vuonna 1989 ilmestyi 40:nnen Jukolan jälkeen Matti Salmenkylän toimittamana mainio Jukolan viestien historiateos ’Jukolan viestin tarina 1949-1988’. Siinä kerrotaan mm. viestien perustamisesta, alkuvaiheista, tärkeistä henkilöistä matkan varrella ja tietysti kustakin viestistä parhaine tuloksineen, kuvineen ja karttoineen. Nyt tehty ’jatko-osa’ kuvaa lähinnä kirjan jälkeisiä vuosia. Koska toivomuksia kirjankin vuosien lisäkartoista ja kaikista tuloksista on tullut runsaasti, on tähän pyritty vastaamaan. Aivan kaikkia tuloksia ei kuitenkaan alkuvuosilta ole löytynyt. Näiden lisäksi sivuilla esitellään jälleen eräitä avainhenkilöitä sekä esitettyjen toiveiden mukaisesti myös eräitä tausta-asioita. Viestien kuvauksissa kilpailujen selostusten osuus on kirjaa laajempi, koska 1 sivun tilarajoitusta ei ole. Jatko-osa ilmestyy kirjanomaisena netti-versiona. Siitä jokainen voi vapaasti tulostaa itse ei-kaupallisiin tarkoituksiin joko kaikki tai vain haluamansa osat ja vaikka sidotuttaa ne kirjaksi. Tähän on päädytty lähinnä taloudellisista syistä. Painettuna Tarina II:n kustannus olisi noussut niin korkeaksi erittäinkin, kun kokemusten valossa sen painos olisi ollut pieni, että sellaisen tekeminen ei ollut järkevää. Projekti on kaikkiaan kestänyt kolmisen vuotta. Kun aiotut ammattikirjoittajat eivät lopulta odottelujen jälkeenkään kiireiltään ehtineet kilpailukuvauksia kirjoittaa eikä vuoden -

The AUCKLAND
The ORIENTEER AUCKLAN D SEPTEMBER 03 It's 'Roo Hunting Season Again Auckland Region Orienteering Maps NorthWest Orienteering Club Auckland Orienteering Club Kaipara Knolls Counties-Manukau Orienteering Club National Orienteering Squad Wounded Knee Mount Auckland Warkworth Woodcocks Snells Beach Shelly Waterfalls Beach Wilson Rood Ahuroa Slater Road Puhoi Turkey Ridge Parakai Waiwera Otakanini Topu Te Heke Orewa Spaghetti Soup Pot Helensville Stag's Roar Luck Whangaparaoa Goblin Country Bees Whose Game Beautiful Hills Knees Shakespear Percy's Delight Waimauku Pulpit Rock Huapai Long Bay Muriwai Albany Kumeu Muriwai Beach Moire Park Onepoto BethellsBeach Motutapu Piha Devonport Karekar Huiea Karamatura Duder's Awhitu Whitford Self's Farm Matakawau Totara Park Pollok Clevedon Kawakawa Bay Papakura Orere Reeve's Farm Drury Hunua Paerata Taurangaruru Ramarama Waiuku Pukekohe Karioitahi Bombay Whiriwhiri South Tuakau Pokeno Mangatawhiri Waiuku Harker Reserve Mercer MARK'S Onewhero Four MAPS '02 Port Waikato Seasons Miranda Meremere Last updated August 2002 Huriwai Contents Regulars Features Page Page Editorial 3 5 Noticeboard Event Calendar 4 12 NZOF Technical Committee Proposal Lisa's North West News 6 Changes to M/W16 Grades Counties Manukau Connection 6 15 JWOC Report Auckland Chatter 7 16 Entry Forms and Registrations NZOF News 9 20 OY Results International O News 9 From the Archives 11 NWOC www.geocities.com/nwocnz AOC http://auckoc.tripod.com OY results http://homepages.paradise.net.nz/pebble/orienteering/ Orienteering news www.maptalk.co.nz NZOF homepage www.nzorienteering.com Sports photos http://communities.msn.co.nz/actionshotz MTB-orienteeering www.mapsport.co.nz/mtbo/mtbo.html Rogaining www.mapsport.co.nz/rog/rogaine.html Ski -orienteering www.mapsport.co.nz/skio/skio.html The Auckland Orienteer is the magazine of the Auckland Orienteering Association and incorporates articles from the Auckland Orienteering Club Inc., the North West Orienteering Club Inc., and the Counties-Manukau Orienteering Club. -

Sesongsboka 2014
! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! Denne boka er tenkt til alle medlemmene i Bækkelagets o-gruppe. Hensikten er å informere medlemmene om hva som skjer i klubbregi sesongen 2014. Vi vil her presentere foreløpige treningsplaner for sesongen, samt klubbens opplegg på de ulike arrangementene og samlingene. I tillegg har vi satt opp en dugnadsliste. Det er også en oversikt over hvilke personer man skal kontakte dersom man har spesifikke spørsmål rettet mot spesielle oppgaver. Dette vil være foreløpige planer og endringer kan tilkomme. Det er derfor viktig å følge med på nyheter på hjemmesiden (bsk-orientering.com) og informasjon i ukesmailen. Bækkelagets sportsklubb sin o-gruppe ønsker å gi et godt tilbud til alle sine medlemmer, både til yngre og eldre, mosjonister og eliteløpere. I dag jobber vi aktivt med rekrutteringsarbeid samtidig som vi ønsker å tilrettelegge for eliteløpere med toppambisjoner. Hver uke sendes en informasjonsmail ut av sportslig leder, Simen, og vi oppfordrer alle medlemmer til å lese denne. Dersom du ikke er på denne mail-listen, ta selv kontakt med Simen på mail ([email protected]). Vi oppfordrer alle medlemmene også til å sjekke Eventor jevnlig for frister til ulike arrangementer i klubbregi. Håper så mange som mulig kan stille på Årsmøte 5. mars 2014 – her er det muligheter for å påvirke klubbens arbeid videre. Hilsen Styret Side Historien om BSK orientering s. 4 Kontaktinformasjon s. 5 Dugnadsliste 2014 s. 6-7 Informasjon om Norway Cup s. 8 Informasjon om materiell s. 9 Fellesinformasjon Tøybestilling s. 10 Prioriterte stafetter 2014 s. 11 Samlinger 2014 s. 12-13 ”Sprek i nærområdet” 2014 s. 17 BSK by Night 2014 s. -

World Championships in Park Orienteering!
Park PWTWorld Tour NOW NEWS NOW PWT at the Olympics tions and see what it looks like", was nese orienteers, officials and media Samaranch's answer. representatives. Two hundred students Park The Park World Tour President, Anders Sepp Hartinger, in charge of the Park will run the whole 5 days of the O- World Tour Vestergård, is spending half of the year World Tour Champions' Week in Au- Ringen, while the officials will complete PWT NOW 2000 in Sydney, Australia. Based at the gust, is NOW! sending the IOC Presi- at least one race. Orienteering and OFFICIAL NEWSLETTER OF THE PARK WORLD TOUR SUPPORTERS´ CLUB • ISSUE 1/2000, APRIL Centre for Olympic Studies, University dent an invitation. The main officials mapping education, business presenta- of New South Wales, Anders is working of the Olympic Committees in Austria tions, cultural events - also with the on his Masters thesis in Social Sciences and its neighbouring countries are also Chinese performing - and tourism are entitled "What is required from a sport sure to be invited. all included in the visitors' programme, in order to be admitted to the Olympic and will be organised in co-operation PWT heads for official status in 2001 Programme?". He is NOW! studying with local universities, authorities and the formal and informal criteria requi- companies. World Champion Jörgen red for inclusion in the Olympic pro- Four babies - and at least Mårtensson and his PWT China com- gramme, and focusing on the two new two weddings! panion Gåvert Wååg will be acting as hosts for the guests from China, where World Championships sports at the Sydney Games: tae kwon- do and triathlon. -

The Auckland Blabbermouth
THE AUCKLAND BLABBERMOUTH OCTOBER 1998 HORSFORD HEATH Scale 1:10000 The Monthly Newsletter for the Auckland area orienteering clubs: Waikato and Auckland Campus Orienteers Auckland Orienteering Club NorthWest Orienteering Club Counties Manukau Orienteering Club SHAUN'S SAY Well, that month flew by. No sooner had I finished the September Issue and I had to start the next one. This magazine is very big, and full of heaps of different articles. Thank you to all those that have contributed, keep it up! The reason for the large size of this mag is due to these people's contributions. It makes my job a breeze. In this issue there is a special report on the World Cup series that has recently finished. One of the reasons that the magazine is a little late is because I was waiting for the last event to happen so that I could include the results. The team was very good at keeping everyone informed of how they were going by sending regular emails to a huge list of people. The report in this mag is snippets of these reports for those that haven't received the emails. I managed to stir up a bit of a wasp nest with my editorial in the last magazine, where I questioned the need to fly NZOF executives around the country for meetings. Included in this mag is the NZOF reply to my questions. Did you all see the orienteering on TV3 last Thursday. WACO was the club of the week on the programme called The Game. Some more good exposure for orienteering. -

Læsning I Områderne Skab
Dansk sølv ved EOC 10 DM kort en barsk 18 barsk omgang Flere unge kan 27 tegne kort 1. årgang Nr.4 August 2004 Dansk Orienterings Forbund 2 Dansk Orienterings- Forbund Medlem af D.I.F og I.O.F Internet-hjemmeside: www.dk.orientering.org/ Kontor: Ole Husen Charlotte Bergmann Hansen Team Danmarks Elitecenter Ryttergårdsvej 118 . 3520 Farum DOF nu og her Ekspeditionstid: blevet guld som ingen medalje. Det mange muligheder i at søge sam- Mandag-fredag..............8.30-12.00 Der var specielt op- muntring at hente var herligt, at det lykkedes. Men re- arbejde og støtte i DIF og andre in- Tlf. ...............................4345 7730 sultaterne set som helhed på her- stitutioner, der arbejde med fritids- [email protected] ved, at de “nye” kla- residen var flotte.Vi oplevede man- og friluftsaktiviteter, men det kræ- rede sig godt ved eu- ge nærved og næsten. Der var spe- ver en løbende og aktiv indsats, Formand: cielt opmuntring at hente ved, at de som kun kan udføres med lønnet Ole Husen ropamesterskaberne “nye” klarede sig godt.Der er et gli- arbejdskraft. Enghave 28, 2960 Rungsted på Sjælland dende generationsskifte i gang. Vi Tlf. arbejde ..................4557 0777 har stadig brug for de rutinerede Vi har tilsyneladende stoppet med- Tlf. privat ......................4586 4416 kræfter, men fremtiden tegner lemsnedgangen. Det gælder nu om Fax ...............................4586 6782 Af Ole Husen, formand for godt. Damerne gjorde måske ikke at få de nye idéer, som udviklings- Dansk Orienterings-Forbund E-mail [email protected] så meget væsen af sig, men det er gruppen kommer med, udmøntet i stadig en ung gruppe, der skal bære praksis så hurtigt som muligt, så det Så er det overstået – vel overstået. -
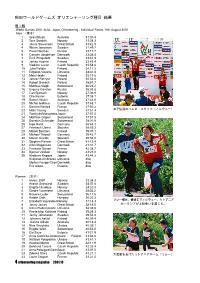
橡 04 Wg Result
秋田ワールドゲームズ オリエンテーリング種目 結果 個人戦 World Games 2001 Akita, Japan Orienteering - Individual Races 18th August 2001 Men (男子) 1 Grant Bluett Australia 31:00.4 2 Tore Sandvik Norway 31:38.3 3 Jamie Stevenson Great Britain 31:42.9 4 Niclas Jonasson Sweden 31:49.7 5 Pavel Naumov Russia 33:17.7 6 Carsten Jorgensen Denmark 33:36.4 7 Emil Wingstedt Sweden 33:41.9 8 Jarkko Huovila Finland 33:43.4 9 Vladimir Lucan Czech Republic 33:58.8 10 Juha Peltola Finland 34:11.3 11 Edgaras Voveris Lithuania 34:31.8 12 Mats Haldin Finland 35:19.8 13 Janusz Porzycz Poland 35:52.0 14 Robert Banach Poland 35:57.7 15 Matthias Niggli Switzerland 36:26.2 16 Evgeny Gavrilov Russia 36:33.8 17 Carl Bjorseth Norway 37:06.9 18 Olle Karner Estonia 37:08.1 19 Robert Walter Australia 37:44.0 20 Michal Jedlicka Czech Republic 37:48.1 21 Damien Renard France 37:50.2 22 Mats Troeng Sweden 37:51.4 女子優勝のハンネ・スタッフ(ノルウェー) 23 Toshiyuki Matsuzawa Japan 37:57.0 24 Matthias Gilgien Switzerland 37:57.5 25 Donatus Schnyder Switzerland 38:01.6 26 Ingo Horst Germany 38:44.1 27 Frantisek Libant Slovakia 38:55.3 28 Mikael Bostrom Finland 39:07.1 29 Michael Thierolf Germany 39:42.7 30 Marian Davidik Slovakia 39:54.0 31 Stephen Palmer Great Britain 41:12.4 32 Allan Mogensen Denmark 41:31.7 33 Francois Gonon France 42:38.7 34 Bjornar Valstad Norway 43:23.0 35 Hirobumi Kagaya Japan 43:44.2 Svajunas Ambrazas Lithuania dsq Morten Fenger-Gron Denmark dsq Eric Aibast Estonia dsq Women (女子) 1 Hanne Staff Norway 33:38.3 2 Anette Granstedt Sweden 34:07.8 3 Birgitte Husebye Norway 34:20.9 4 Giedre Voveriene -

Soon a World Champ?!
Park PWTWorld Tour NOW NEWS NOW Park Orienteering World Championships Park PWTWorld Tour NOW We say Yes! OFFICIAL NEWSLETTER OF THE PARK WORLD TOUR SUPPORTERS´ CLUB • ISSUE 2/2000, JULY The World's Best Orienteers Tellesbø and Mikkola PWT première winners Hanne Staff, World Champion, European Champion, World Cup and PWT Winner: "A World Champion- ships series in Park Orienteering will Soon a World Champ?! increase the interest for our sport world- wide", says Hanne – the newly-crowned Odin Tellesbø of Norway was – like Finland's Marika Mikkola – a happy Towards the 2008 Olympics European Champion and leading lady on the international orienteering scene surprise winner at the Park World Tour 2000 première. Already in 2001 One of the Champions' Week for the last four years. A successful and he may be able to compete for the World Championship title in Park participants will be Wang Junxia of experienced all-round orienteer, she does China, the reigning Olympic 5,000 not want to see the park distance Orienteering. A decision to introduce an official Park Orienteering World metres Champion and world record included in the classic World Championships can be made by the IOF Congress, which will be held in holder at 3,000 and 10,000 metres. Championships week. Egil Johansen (far left) ran the long night leg in the team made up of former She will make her elite orienteering "It should be a separate series of 5-6 Austria, 1-5 August, at the same time as the PWT Champions' Week gathers debut at the PWT race in Sweden on races. -

Resultater 2017
H A M A R O R I E N T E R I N G S K L U B B RESULTATER 2017 Her finner du resultater fra alle enkeltløp som har hatt deltakelse fra Hamar OK i løpet av året 2017. Ta kontakt med Tærje hvis du har vært med på løp som ikke er med i resultatoversikten, eller oppdager feil. Epost: tegudbra(at)online.no Resultatene er forsøkt satt opp i denne rekkefølge: E-, A- B- C- og N-klasser 11. jan., Vang OL 22. jan., Nordmarka Skiorienteringsklubb Vintercup #2 SKI-O, O-treff Nærløp, natt Nasjonalt renn, langdistanse H-Lang 6,45 km 4 startet D15-16 5,01 km 16 startet 1 Ove Arntvord Haugereid, Løten OL 1:01:50 1 Ane Sofie Krogh, Oppsal IF 30:24 Sveinung Syversen disket 6 Kamilla Lome 37:06 D17-20 6,53 km 23 startet 14. jan., Ås-NMBU 1 Jenny Baklid, Konnerud IL 41:38 Ås by Night & Fog Cup #4 8 Anine Lome 47:17 Nærløp, mellomdistanse D21- 8,18 km 7 startet H17-AK 4,8 km 58 startet 1 Evine Westli Andersen, Løten OL 45:52 1 Jan Richard Eriksen, Asker SK 39:18 3 Stine Olsen Kirkevik 47:19 24 Terje Gudbrandsen 51:41 D40- 5,95 km 12 startet 1 Valborg Madslien, Lillehammer OK 35:52 21. jan., Nordmarka Skiorienteringsklubb 6 Mona Lome 48:30 SKI-O, O-treff H11-12 3,24 km 7 startet Nasjonalt renn, mellomdistanse 1 Rasmus Eastwood, Lillomarka OL 22:40 D15-16 3,90 km 15 startet 6 Eirik Olsen Kirkevik 39:54 1 Ane Sofie Krogh, Oppsal IF 27:14 15 Kamilla Lome 41:19 29. -

Wingstedt Upp I Delad Världscupledning
2004-10-19 16:47 CEST Wingstedt upp i delad världscupledning Emil Wingstedt delar ledningen i världscupen när två deltävlingar återstår. Visserligen blev svenskan bara 13:e man i tisdagseftermiddagens sprint i Dresden, men värste konkurrenten, fransmannan Thierry Gueorgiou, slutade 17:e och därmed är det helt lika mellan de båda. Emil Wingstedts förkylning förvärrades inte av förmiddagens kval och därmed kom han till start i sprinttävlingen, och det kan visa sig vara värdefullt när världscupen summeras på lördag. Nu delar han nämligen ledningen med Thierry Gueorgiou på 160 poäng, elva poäng före norske sprintvinnaren Öystein Kvaal Österbö. Norrmannen Österbö vann sprinten med 13 sekunders marginal till Mikkel Lund, Danmark, och Daniel Hubmann, Schweiz, vilka delade andraplatsen. Världsmästaren på distansen, Niclas Jonasson, blev bäste svensk på sjätte plats. I damtävlingen vann Simone Niggli-Luder, världscupledarinnan från Schweiz, som har maximala 200 poäng i världscupen. Men det var svenskt bakom, och precis som på sprinten i VM tog Karolina A Höjsgaard andraplatsen. Dessutom blev Emma Engstrand trea. I världscupsammandraget parkerar svenskorna på tredje respektive fjärde plats. Resultat, världscupen sprint, Dresden 1 Øystein Kvaal Østerbø NOR 13:25 2 Mikkel Lund DEN 13:38 2 Daniel Hubmann SUI 13:38 4 Marten Bostrom FIN 13:49 5 Andrey Khramov RUS 13:50 6 Niclas Jonasson SWE 13:53 7 Holger Hott Johansen NOR 13:55 8 Damien Renard FRA 13:57 9 Mats Haldin FIN 13:59 10 Michele Tavernaro ITA 14:11 11 Nicolas Girsch FRA 14:12 12 Christian Ott -

Orienteering Marathons - a Developing Discipline
ORIENTEERING CANADA Published by the Canadian Orienteering Federation Box 62052. Convent Glen P.O. Orleans, Ontario, K1C 7H8 E-MAIL [email protected] Tel: (613) 830-1147 FAX: (613) 830-0456 OFFICIAL NEWSLETTER OF THE CANADIAN ORIENTEERING FEDERATION Vol. 28 No. 3 FALL 1999 ISSN 0227-6658 CONTENTS MODERN TECHNOLOGIES AND Front Cover 1 ORIENTEERING Land Permission 1 Modem Technologies 1 Wide spread use of computers and modern technologies AGM - Board Activity 2 have greatly affected every area of the workplace, home Richest Prize in Sight 3 life, recreation and lifestyles. Many time consuming manual Promotion - Newspapers, Radio, Internet 4 tasks have been converted to computers: e.g. administration, Electronic punching of course 5-6 meet organization and map production. Canadian Championships Registration Forms 7-10 Orienteering Marathon - a developing discipline 11 Modern technologies have also had a great impact in Internet and email 12 Elite News 13-14 orienteering. Most federations and clubs developed web Yukon News 14 sites that provide information on membership, meet Sale Items 15 schedules, results, contact names, telephone numbers, email COF and Association Contacts 16 addresses and other items. A prime example of computer technology in orienteering is the OCAD (0-map Computer Assisted Drawing) program, developed by Hans Steinegger (Switzerland). The first LAND PERMISSION version of OCAD, released in 1990, was quickly accepted by 0-mappers and within a few years the majority of 0- A Landowner Permission Guide is being prepared to assist maps were OCAD prepared. clubs and association in negotiations with land owners, property managers, park and conservation authorities in A recent innovation is electronic punching. -

ÅRSBERETNING 2016–2017 Forbundsting, Trondheim 10.–11
ÅRSBERETNING 2016–2017 Forbundsting, Trondheim 10.–11. mars 2018 INNHOLD 1 NORGES ORIENTERINGSFORBUNDS TILLITSVALGTE .......................................................... 3 2 FASTE OG DELTIDSANSATTE .................................................................................................. 3 3 REPRESENTASJON ................................................................................................................... 5 4 STYRET ...................................................................................................................................... 5 Styrets vurdering av perioden 2016-2017 ................................................................................ 5 Hedersbevisninger/priser .......................................................................................................... 7 Regnskap og årsberetning ........................................................................................................ 8 5 KLUBBSERVICE ......................................................................................................................... 9 Lags- og aktivitetstall ............................................................................................................... 9 Arbeid mot 1%-målet i Strategi 2020 .................................................................................... 10 Kompetanse ............................................................................................................................ 11 Andre prosjekter og samlinger ..............................................................................................