Nickel on Mars: Constraints on Meteoritic Material at the Surface A
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Copyrighted Material
Index Abulfeda crater chain (Moon), 97 Aphrodite Terra (Venus), 142, 143, 144, 145, 146 Acheron Fossae (Mars), 165 Apohele asteroids, 353–354 Achilles asteroids, 351 Apollinaris Patera (Mars), 168 achondrite meteorites, 360 Apollo asteroids, 346, 353, 354, 361, 371 Acidalia Planitia (Mars), 164 Apollo program, 86, 96, 97, 101, 102, 108–109, 110, 361 Adams, John Couch, 298 Apollo 8, 96 Adonis, 371 Apollo 11, 94, 110 Adrastea, 238, 241 Apollo 12, 96, 110 Aegaeon, 263 Apollo 14, 93, 110 Africa, 63, 73, 143 Apollo 15, 100, 103, 104, 110 Akatsuki spacecraft (see Venus Climate Orbiter) Apollo 16, 59, 96, 102, 103, 110 Akna Montes (Venus), 142 Apollo 17, 95, 99, 100, 102, 103, 110 Alabama, 62 Apollodorus crater (Mercury), 127 Alba Patera (Mars), 167 Apollo Lunar Surface Experiments Package (ALSEP), 110 Aldrin, Edwin (Buzz), 94 Apophis, 354, 355 Alexandria, 69 Appalachian mountains (Earth), 74, 270 Alfvén, Hannes, 35 Aqua, 56 Alfvén waves, 35–36, 43, 49 Arabia Terra (Mars), 177, 191, 200 Algeria, 358 arachnoids (see Venus) ALH 84001, 201, 204–205 Archimedes crater (Moon), 93, 106 Allan Hills, 109, 201 Arctic, 62, 67, 84, 186, 229 Allende meteorite, 359, 360 Arden Corona (Miranda), 291 Allen Telescope Array, 409 Arecibo Observatory, 114, 144, 341, 379, 380, 408, 409 Alpha Regio (Venus), 144, 148, 149 Ares Vallis (Mars), 179, 180, 199 Alphonsus crater (Moon), 99, 102 Argentina, 408 Alps (Moon), 93 Argyre Basin (Mars), 161, 162, 163, 166, 186 Amalthea, 236–237, 238, 239, 241 Ariadaeus Rille (Moon), 100, 102 Amazonis Planitia (Mars), 161 COPYRIGHTED -

Planetary Report Report
The PLANETARYPLANETARY REPORT REPORT Volume XXIX Number 1 January/February 2009 Beyond The Moon From The Editor he Internet has transformed the way science is On the Cover: Tdone—even in the realm of “rocket science”— The United States has the opportunity to unify and inspire the and now anyone can make a real contribution, as world’s spacefaring nations to create a future brightened by long as you have the will to give your best. new goals, such as the human exploration of Mars and near- In this issue, you’ll read about a group of amateurs Earth asteroids. Inset: American astronaut Peggy A. Whitson who are helping professional researchers explore and Russian cosmonaut Yuri I. Malenchenko try out training Mars online, encouraged by Mars Exploration versions of Russian Orlan spacesuits. Background: The High Rovers Project Scientist Steve Squyres and Plane- Resolution Camera on Mars Express took this snapshot of tary Society President Jim Bell (who is also head Candor Chasma, a valley in the northern part of Valles of the rovers’ Pancam team.) Marineris, on July 6, 2006. Images: Gagarin Cosmonaut Training This new Internet-enabled fun is not the first, Center. Background: ESA nor will it be the only, way people can participate in planetary exploration. The Planetary Society has been encouraging our members to contribute Background: their minds and energy to science since 1984, A dust storm blurs the sky above a volcanic caldera in this image when the Pallas Project helped to determine the taken by the Mars Color Imager on Mars Reconnaissance Orbiter shape of a main-belt asteroid. -

The Evolution of a Heterogeneous Martian Mantle: Clues from K, P, Ti, Cr, and Ni Variations in Gusev Basalts and Shergottite Meteorites
Earth and Planetary Science Letters 296 (2010) 67–77 Contents lists available at ScienceDirect Earth and Planetary Science Letters journal homepage: www.elsevier.com/locate/epsl The evolution of a heterogeneous Martian mantle: Clues from K, P, Ti, Cr, and Ni variations in Gusev basalts and shergottite meteorites Mariek E. Schmidt a,⁎, Timothy J. McCoy b a Dept. of Earth Sciences, Brock University, St. Catharines, ON, Canada L2S 3A1 b Dept. of Mineral Sciences, National Museum of Natural History, Smithsonian Institution, Washington, DC 20560-0119, USA article info abstract Article history: Martian basalts represent samples of the interior of the planet, and their composition reflects their source at Received 10 December 2009 the time of extraction as well as later igneous processes that affected them. To better understand the Received in revised form 16 April 2010 composition and evolution of Mars, we compare whole rock compositions of basaltic shergottitic meteorites Accepted 21 April 2010 and basaltic lavas examined by the Spirit Mars Exploration Rover in Gusev Crater. Concentrations range from Available online 2 June 2010 K-poor (as low as 0.02 wt.% K2O) in the shergottites to K-rich (up to 1.2 wt.% K2O) in basalts from the Editor: R.W. Carlson Columbia Hills (CH) of Gusev Crater; the Adirondack basalts from the Gusev Plains have more intermediate concentrations of K2O (0.16 wt.% to below detection limit). The compositional dataset for the Gusev basalts is Keywords: more limited than for the shergottites, but it includes the minor elements K, P, Ti, Cr, and Ni, whose behavior Mars igneous processes during mantle melting varies from very incompatible (prefers melt) to very compatible (remains in the shergottites residuum). -

Legalizing Marijuana: California's Pot of Gold?
University of the Pacific Scholarly Commons McGeorge School of Law Scholarly Articles McGeorge School of Law Faculty Scholarship 2009 Legalizing Marijuana: California’s Pot of Gold? Michael Vitiello Pacific cGeM orge School of Law Follow this and additional works at: https://scholarlycommons.pacific.edu/facultyarticles Part of the Criminal Law Commons Recommended Citation Michael Vitiello, Legalizing Marijuana: California’s Pot of Gold?, 2009 Wis. L. Rev. 1349. This Article is brought to you for free and open access by the McGeorge School of Law Faculty Scholarship at Scholarly Commons. It has been accepted for inclusion in McGeorge School of Law Scholarly Articles by an authorized administrator of Scholarly Commons. For more information, please contact [email protected]. ESSAY LEGALIZING MARUUANA: CALIFORNIA'S POT OF GOLD? MICHAEL VITIELLO* In early 2009, a member of the California Assembly introduced a bill that would have legalized marijuana in an effort to raise tax revenue and reduce prison costs. While the bill's proponent withdrew the bill, he vowed to renew his efforts in the next term. Other prominent California officials, including Governor Schwarzenegger, have indicated their willingness to study legalization in light of California's budget shortfall. For the first time in over thirty years, politicians are giving serious consideration to a proposal to legalize marijuana. But already, the public debate has degenerated into traditional passionate advocacy, with ardent prohibitionists raising the specter of doom, and marijuana advocates promising billions of dollars in tax revenues and reduced prison costs. Rather than rehashing the old debate about legalizing marijuana, this Essay offers a balanced view of the proposal to legalize marijuana, specifically as a measure to raise revenue and to reduce prison costs. -

Planting and Germination of Sweet Potato, Yam and Radish Plants in the Mars Desert Research Station
Copyright © 2015 by Olenka Jibaja Valderrama. Published by The Mars Society with permission. Planting and Germination of Sweet Potato, Yam and Radish Plants in the Mars Desert Research Station Olenka Jibaja Valderrama Universidad Católica Santo Toribio de Mogrovejo – Chiclayo, Perú [email protected] Abstract: As part of the future exploration of the universe, manned missions to other planets or satellites near Earth will be needed. One of the main objectives of these missions will be to reach Mars because it is at a relatively close distance to our planet and because both planets have some common characteristics. Since the economic investment of these explorations is quite high, the duration of them must be significant; that’s why the missions should aim to be self-sufficient. The search for food sources for crew members is a key factor to be investigated and even more attention should be on food whose production is possible and sustainable in the environment where the mission arises. This work was involved with the planting and germination of radish, yam and sweet potato plants in the greenhouse of the Mars Desert Research Station (MDRS) and the main objective was to investigate the characteristics of growth and development of these plants in Martian conditions to find out whether its production is possible or not. Orange skin and white skin: two types of sweet potatoes were planted. During rotation, it was discovered that yam grew faster under these conditions than the rest of the tubers, although its development was slower as compared to terrestrial conditions. Radish plants in Mars regolith grew in a similar speed than in the fertile Earth soil, and even faster than in drier soil taken from the desert. -

Open Research Online Oro.Open.Ac.Uk
Open Research Online The Open University’s repository of research publications and other research outputs Centimeter to decimeter hollow concretions and voids in Gale Crater sediments, Mars Journal Item How to cite: Wiens, Roger C.; Rubin, David M.; Goetz, Walter; Fairén, Alberto G.; Schwenzer, Susanne P.; Johnson, Jeffrey R.; Milliken, Ralph; Clark, Ben; Mangold, Nicolas; Stack, Kathryn M.; Oehler, Dorothy; Rowland, Scott; Chan, Marjorie; Vaniman, David; Maurice, Sylvestre; Gasnault, Olivier; Rapin, William; Schroeder, Susanne; Clegg, Sam; Forni, Olivier; Blaney, Diana; Cousin, Agnes; Payré, Valerie; Fabre, Cecile; Nachon, Marion; Le Mouelic, Stephane; Sautter, Violaine; Johnstone, Stephen; Calef, Fred; Vasavada, Ashwin R. and Grotzinger, John P. (2017). Centimeter to decimeter hollow concretions and voids in Gale Crater sediments, Mars. Icarus, 289 pp. 144–156. For guidance on citations see FAQs. c 2017 Elsevier Inc. https://creativecommons.org/licenses/by-nc-nd/4.0/ Version: Accepted Manuscript Link(s) to article on publisher’s website: http://dx.doi.org/doi:10.1016/j.icarus.2017.02.003 Copyright and Moral Rights for the articles on this site are retained by the individual authors and/or other copyright owners. For more information on Open Research Online’s data policy on reuse of materials please consult the policies page. oro.open.ac.uk Centimeter to Decimeter Hollow Concretions and Voids In Gale Crater Sediments, Mars Roger C. Wiens1, David M. Rubin2, Walter Goetz3, Alberto G. Fairén4, Susanne P. Schwenzer5, Jeffrey R. Johnson6, Ralph Milliken7, Ben Clark8, Nicolas Mangold9, Kathryn M. Stack10, Dorothy Oehler11, Scott Rowland12, Marjorie Chan13, David Vaniman14, Sylvestre Maurice15, Olivier Gasnault15, William Rapin15, Susanne Schroeder16, Sam Clegg1, Olivier Forni15, Diana Blaney10, Agnes Cousin15, Valerie Payré17, Cecile Fabre17, Marion Nachon18, Stephane Le Mouelic9, Violaine Sautter19, Stephen Johnstone1, Fred Calef10, Ashwin R. -

Chemin X-Ray Diffraction of the Windjana Sample
PUBLICATIONS Journal of Geophysical Research: Planets RESEARCH ARTICLE Mineralogy, provenance, and diagenesis of a potassic 10.1002/2015JE004932 basaltic sandstone on Mars: CheMin X-ray diffraction Special Section: of the Windjana sample (Kimberley area, Gale Crater) The Mars Science Laboratory Rover Mission (Curiosity) at The Allan H. Treiman1, David L. Bish2, David T. Vaniman3, Steve J. Chipera4, David F. Blake5, Kimberley, Gale Crater, Mars Doug W. Ming6, Richard V. Morris6, Thomas F. Bristow5, Shaunna M. Morrison7, Michael B. Baker8, Elizabeth B. Rampe6, Robert T. Downs7, Justin Filiberto9, Allen F. Glazner10, Ralf Gellert11, Key Points: Lucy M. Thompson12, Mariek E. Schmidt13, Laetitia Le Deit14, Roger C. Wiens15, Amy C. McAdam16, • First mineralogical analysis of Cherie N. Achilles2, Kenneth S. Edgett17, Jack D. Farmer18, Kim V. Fendrich7, John P. Grotzinger8, sandstone on Mars 19 20 8 21 12 • Windjana sandstone very rich in Sanjeev Gupta , John Michael Morookian , Megan E. Newcombe , Melissa S. Rice , John G. Spray , sanidine, implying a trachyte source Edward M. Stolper8, Dawn Y. Sumner22, Ashwin R. Vasavada20, and Albert S. Yen20 rock • The source of Gale Crater sediments is 1Lunar and Planetary Institute, Houston, Texas, USA, 2Department of Geological Sciences, Indiana University, Bloomington, Indiana, an incredibly diverse igneous terrane USA, 3Planetary Science Institute,Tucson,Arizona,USA,4Chesapeake Energy Corporation, Oklahoma City, Oklahoma, USA, 5NASA Ames Research Center, Moffett Field, California, USA, 6Astromaterials -
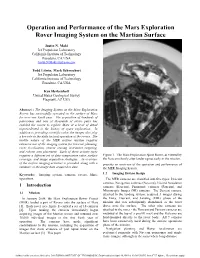
Operation and Performance of the Mars Exploration Rover Imaging System on the Martian Surface
Operation and Performance of the Mars Exploration Rover Imaging System on the Martian Surface Justin N. Maki Jet Propulsion Laboratory California Institute of Technology Pasadena, CA USA [email protected] Todd Litwin, Mark Schwochert Jet Propulsion Laboratory California Institute of Technology Pasadena, CA USA Ken Herkenhoff United States Geological Survey Flagstaff, AZ USA Abstract - The Imaging System on the Mars Exploration Rovers has successfully operated on the surface of Mars for over one Earth year. The acquisition of hundreds of panoramas and tens of thousands of stereo pairs has enabled the rovers to explore Mars at a level of detail unprecedented in the history of space exploration. In addition to providing scientific value, the images also play a key role in the daily tactical operation of the rovers. The mobile nature of the MER surface mission requires extensive use of the imaging system for traverse planning, rover localization, remote sensing instrument targeting, and robotic arm placement. Each of these activity types requires a different set of data compression rates, surface Figure 1. The Mars Exploration Spirit Rover, as viewed by coverage, and image acquisition strategies. An overview the Navcam shortly after lander egress early in the mission. of the surface imaging activities is provided, along with a presents an overview of the operation and performance of summary of the image data acquired to date. the MER Imaging System. Keywords: Imaging system, cameras, rovers, Mars, 1.2 Imaging System Design operations. The MER cameras are classified into five types: Descent cameras, Navigation cameras (Navcam), Hazard Avoidance 1 Introduction cameras (Hazcam), Panoramic cameras (Pancam), and Microscopic Imager (MI) cameras. -

Evaluation of Pilot Summer Activities Programme for 16 Year Olds
RESEARCH Evaluation of Pilot Summer Activities Programme for 16 Year Olds Graham Thom SQW Ltd Research Report RR341 Research Report No 341 Evaluation of Pilot Summer Activities Programme for 16 Year Olds Graham Thom SQW Ltd The views expressed in this report are the authors' and do not necessarily reflect those of the Department for Education and Skills. © Queen’s Printer 2002. Published with the permission of DfES on behalf of the Controller of Her Majesty's Stationery Office. Applications for reproduction should be made in writing to The Crown Copyright Unit, Her Majesty's Stationery Office, St Clements House, 2-16 Colegate, Norwich NR3 1BQ. ISBN 1 84185 734 3 June 2002 Table of Contents Acknowledgements Executive Summary Chapter Page 1 Introduction 1 The role of Connexions 1 Methodology 2 Report structure 3 2 Characteristics of pilot projects 4 Describing the partnerships 4 Management structure 7 Other staff involved in the partnerships 11 Engaging young people 14 Delivering the project 19 Summary 29 3 Characteristics of participants 30 Personal characteristics 30 Academic performance 32 Levels of personal social development 35 Future plans 36 Comparing the profile of participants with the 2000 37 cohort Getting involved 38 Summary 41 4 The impact of the programme 42 Overall impact on future plans 42 Overall impact on personal and social characteristics 45 Overall satisfaction with the programme 46 Impact on different groups 46 Key influencing variables 49 Impact on young people – the longer term perspective 51 Summary 56 5 Conclusions -

Volcanism on Mars
Author's personal copy Chapter 41 Volcanism on Mars James R. Zimbelman Center for Earth and Planetary Studies, National Air and Space Museum, Smithsonian Institution, Washington, DC, USA William Brent Garry and Jacob Elvin Bleacher Sciences and Exploration Directorate, Code 600, NASA Goddard Space Flight Center, Greenbelt, MD, USA David A. Crown Planetary Science Institute, Tucson, AZ, USA Chapter Outline 1. Introduction 717 7. Volcanic Plains 724 2. Background 718 8. Medusae Fossae Formation 725 3. Large Central Volcanoes 720 9. Compositional Constraints 726 4. Paterae and Tholi 721 10. Volcanic History of Mars 727 5. Hellas Highland Volcanoes 722 11. Future Studies 728 6. Small Constructs 723 Further Reading 728 GLOSSARY shield volcano A broad volcanic construct consisting of a multitude of individual lava flows. Flank slopes are typically w5, or less AMAZONIAN The youngest geologic time period on Mars identi- than half as steep as the flanks on a typical composite volcano. fied through geologic mapping of superposition relations and the SNC meteorites A group of igneous meteorites that originated on areal density of impact craters. Mars, as indicated by a relatively young age for most of these caldera An irregular collapse feature formed over the evacuated meteorites, but most importantly because gases trapped within magma chamber within a volcano, which includes the potential glassy parts of the meteorite are identical to the atmosphere of for a significant role for explosive volcanism. Mars. The abbreviation is derived from the names of the three central volcano Edifice created by the emplacement of volcanic meteorites that define major subdivisions identified within the materials from a centralized source vent rather than from along a group: S, Shergotty; N, Nakhla; C, Chassigny. -

AST-2018-1847-Ver9-Hughes 4P 260..283
ASTROBIOLOGY Volume 19, Number 3, 2019 Mary Ann Liebert, Inc. DOI: 10.1089/ast.2018.1847 Basaltic Terrains in Idaho and Hawai‘i as Planetary Analogs for Mars Geology and Astrobiology Scott S. Hughes,1 Christopher W. Haberle,2 Shannon E. Kobs Nawotniak,1 Alexander Sehlke,3 W. Brent Garry,4 Richard C. Elphic,3 Samuel J. Payler,5 Adam H. Stevens,5 Charles S. Cockell,5 Allyson L. Brady,6 Jennifer L. Heldmann,3,7 and Darlene S.S. Lim3,8 Abstract Field research target regions within two basaltic geologic provinces are described as Earth analogs to Mars. Regions within the eastern Snake River Plain of Idaho and the Big Island of Hawai‘i, the United States, provinces that represent analogs of present-day and early Mars, respectively, were evaluated on the basis of geologic settings, rock lithology and geochemistry, rock alteration, and climate. Each of these factors provides rationale for the selection of specific targets for field research in five analog target regions: (1) Big Craters and (2) Highway lava flows at Craters of the Moon National Monument and Preserve, Idaho, and (3) Mauna Ulu low shield, (4) Kılauea Iki lava lake, and (5) Kılauea caldera in the Kılauea Volcano summit region and the East Rift Zone of Hawai‘i. Our evaluation of compositional and textural attributes, as well as the effects of syn- and posteruptive rock alteration, shows that basaltic terrains in Idaho and Hawai‘i provide a way to characterize the geology and major geologic substrates that host biological activity of relevance to Mars exploration. This work provides the foundation to better understand the scientific questions related to the habitability of basaltic terrains, the rationale behind selecting analog field targets, and their applicability as analogs to Mars. -

Mineralogy of the Martian Surface
EA42CH14-Ehlmann ARI 30 April 2014 7:21 Mineralogy of the Martian Surface Bethany L. Ehlmann1,2 and Christopher S. Edwards1 1Division of Geological & Planetary Sciences, California Institute of Technology, Pasadena, California 91125; email: [email protected], [email protected] 2Jet Propulsion Laboratory, California Institute of Technology, Pasadena, California 91109 Annu. Rev. Earth Planet. Sci. 2014. 42:291–315 Keywords First published online as a Review in Advance on Mars, composition, mineralogy, infrared spectroscopy, igneous processes, February 21, 2014 aqueous alteration The Annual Review of Earth and Planetary Sciences is online at earth.annualreviews.org Abstract This article’s doi: The past fifteen years of orbital infrared spectroscopy and in situ exploration 10.1146/annurev-earth-060313-055024 have led to a new understanding of the composition and history of Mars. Copyright c 2014 by Annual Reviews. Globally, Mars has a basaltic upper crust with regionally variable quanti- by California Institute of Technology on 06/09/14. For personal use only. All rights reserved ties of plagioclase, pyroxene, and olivine associated with distinctive terrains. Enrichments in olivine (>20%) are found around the largest basins and Annu. Rev. Earth Planet. Sci. 2014.42:291-315. Downloaded from www.annualreviews.org within late Noachian–early Hesperian lavas. Alkali volcanics are also locally present, pointing to regional differences in igneous processes. Many ma- terials from ancient Mars bear the mineralogic fingerprints of interaction with water. Clay minerals, found in exposures of Noachian crust across the globe, preserve widespread evidence for early weathering, hydrothermal, and diagenetic aqueous environments. Noachian and Hesperian sediments include paleolake deposits with clays, carbonates, sulfates, and chlorides that are more localized in extent.