Type I Diabetes Treatments Clinical Notes Recommendations
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Treatment of Diabetes Mellitus
TREATMENT OF DIABETES MELLITUS DIABETES is a condition that affects how the body makes energy from food. Food is broken down into sugar (glucose) in the body and released into the blood. When the blood sugar level rises after a meal, insulin responds to let the sugar into the cells to be used as energy. In diabetes, the body either does not make enough insulin or it stops responding to insulin as well as it should. This results in sugar staying in the blood and leads to serious health problems over time. DIAGNOSIS OF DIABETES1 • A1C Test: Lab test measuring average blood sugar over past two to three months • Fasting Blood Sugar Test: Lab test measuring blood sugar after eight hours of no food or drink • Oral Glucose Tolerance Test (OGTT): Measures blood sugar before and two hours after drinking a specific sugary liquid • Random Blood Sugar Test: Measures blood sugar at a moment in time, without any kind of preparation (like fasting) FASTING BLOOD ORAL GLUCOSE TOLERANCE RANDOM BLOOD RESULT A1C TEST SUGAR TEST TEST SUGAR TEST Diabetes ≥ 6.5% ≥126 mg/dL ≥ 200 mg/dL ≥ 200 mg/dL Prediabetes 5.7 – 6.4% 100 – 125 mg/dL 140 – 199 mg/dL N/A Normal < 5.7% ≤99 mg/dL < 140 mg/dL N/A NON-DRUG TREATMENTS2 THERAPY COST WHAT TO EXPECT Diet (Mediterranean diet) and exercise (30 minutes a day, five days a week of moderate- Weight loss $-$$ intensity exercise); 7% weight loss decreases risk of diabetes3 Psychological intervention $$-$$$ Psychotherapy may reduce diabetic distress and improve glycemic control4,5 nationalcooperativerx.com PRESCRIPTION TREATMENTS -

The Antidiabetic Drug Lobeglitazone Has the Potential to Inhibit PTP1B T Activity ⁎ Ruth F
Bioorganic Chemistry 100 (2020) 103927 Contents lists available at ScienceDirect Bioorganic Chemistry journal homepage: www.elsevier.com/locate/bioorg The antidiabetic drug lobeglitazone has the potential to inhibit PTP1B T activity ⁎ Ruth F. Rochaa, Tiago Rodriguesc, Angela C.O. Menegattia,b, , Gonçalo J.L. Bernardesc,d, Hernán Terenzia a Centro de Biologia Molecular Estrutural, Departamento de Bioquímica, Universidade Federal de Santa Catarina, Campus Trindade, 88040-900 Florianópolis, SC, Brazil b Universidade Federal do Piauí, CPCE, 64900-000 Bom Jesus, PI, Brazil c Instituto de Medicina Molecular, Faculdade de Medicina, Universidade de Lisboa, Avenida Professor Egas Moniz, 1649-028 Lisbon, Portugal d Department of Chemistry, University of Cambridge, Lensfield Road, CB2 1EW Cambridge, UK ARTICLE INFO ABSTRACT Keywords: Protein tyrosine phosphatase 1B (PTP1B) is considered a potential therapeutic target for the treatment of type 2 Thiazolidinediones diabetes mellitus (T2DM), since this enzyme plays a significant role to down-regulate insulin and leptin sig- Lobeglitazone nalling and its over expression has been implicated in the development of insulin resistance, T2DM and obesity. PPAR-γ Some thiazolidinediones (TZD) derivatives have been reported as promising PTP1B inhibitors with anti hy- PTP1B perglycemic effects. Recently, lobeglitazone, a new TZD, was described as an antidiabetic drug that targetsthe Non-competitive inhibitors PPAR-γ (peroxisome γ proliferator-activated receptor) pathway, but no information on its effects on PTP1B have been reported to date. We investigated the effects of lobeglitazone on PTP1B activity in vitro. Surprisingly, lobeglitazone led to moderate inhibition on PTP1B (IC50 42.8 ± 3.8 µM) activity and to a non-competitive reversible mechanism of action. -

Supplementary Material
Supplementary material Table S1. Search strategy performed on the following databases: PubMed, Embase, the Cochrane Central Register of Controlled Trials (CENTRAL). 1. Randomi*ed study OR random allocation OR Randomi*ed controlled trial OR Random* Control* trial OR RCT Epidemiological study 2. sodium glucose cotransporter 2 OR sodium glucose cotransporter 2 inhibitor* OR sglt2 inhibitor* OR empagliflozin OR dapagliflozin OR canagliflozin OR ipragliflozin OR tofogliflozin OR ertugliflozin OR sotagliflozin OR sergliflozin OR remogliflozin 3. 1 AND 2 1 Table S2. Safety outcomes of empagliflozin and linagliptin combination therapy compared with empagliflozin or linagliptin monotherapy in treatment naïve type 2 diabetes patients Safety outcome Comparator 1 Comparator 2 I2 RR [95% CI] Number of events Number of events / / total subjects total subjects i. Empagliflozin + linagliptin vs empagliflozin monotherapy Empagliflozin + Empagliflozin linagliptin monotherapy ≥ 1 AE(s) 202/272 203/270 77% 0.99 [0.81, 1.21] ≥ 1 drug-related 37/272 38/270 0% 0.97 [0.64, 1.47] AE(s) ≥ 1 serious AE(s) 13/272 19/270 0% 0.68 [0.34, 1.35] Hypoglycaemia* 0/272 5/270 0% 0.18 [0.02, 1.56] UTI 32/272 25/270 29% 1.28 [0.70, 2.35] Events suggestive 12/272 13/270 9% 0.92 [0.40, 2.09] of genital infection i. Empagliflozin + linagliptin vs linagliptin monotherapy Empagliflozin + Linagliptin linagliptin monotherapy ≥ 1 AE(s) 202/272 97/135 0% 1.03 [0.91, 1.17] ≥ 1 drug-related 37/272 17/135 0% 1.08 [0.63, 1.84] AE(s) ≥ 1 serious AE(s) 13/272 2/135 0% 3.22 [0.74, 14.07] Hypoglycaemia* 0/272 1/135 NA 0.17 [0.01, 4.07] UTI 32/272 12/135 0% 1.32 [0.70, 2.49] Events suggestive 12/272 4/135 0% 1.45 [0.47, 4.47] of genital infection RR, relative risk; AE, adverse event; UTI, urinary tract infection. -

Januvia (Sitagliptin) Tablets
CENTER FOR DRUG EVALUATION AND RESEARCH Approval Package for: APPLICATION NUMBER: NDA 021995/S-013 Trade Name: JANUVIA Generic Name: Sitagliptin Sponsor: Merck & Co., Inc. Approval Date: 12/28/2009 Indications: JANUVIA is indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus. CENTER FOR DRUG EVALUATION AND RESEARCH APPLICATION NUMBER: NDA 021995/S-013 CONTENTS Reviews / Information Included in this NDA Review. Approval Letter X Other Action Letters X Labeling X Summary Review Officer/Employee List Office Director Memo Cross Discipline Team Leader Review Medical Review(s) X Chemistry Review(s) Environmental Assessment Pharmacology Review(s) X Statistical Review(s) Microbiology Review(s) Clinical Pharmacology/Biopharmaceutics Review(s) Risk Assessment and Risk Mitigation Review(s) X Proprietary Name Review(s) Other Review(s) X Administrative/Correspondence Document(s) X CENTER FOR DRUG EVALUATION AND RESEARCH APPLICATION NUMBER: NDA 021995/S-013 APPROVAL LETTER DEPARTMENT OF HEALTH AND HUMAN SERVICES Food and Drug Administration Silver Spring MD 20993 NDA 021995/S-013 SUPPLEMENT APPROVAL Merck & Co., Inc. Attention: Richard J. Swanson, Ph.D. Director, Regulatory Affairs P.O. Box 1000, UG2C-50 North Wales, PA 19454-1099 Dear Dr. Swanson: Please refer to your supplemental new drug application (S-013) dated and received March 5, 2009, submitted under section 505(b) of the Federal Food, Drug, and Cosmetic Act (FDCA) for Januvia (sitagliptin) tablets. We also refer to your supplemental new drug application (b) (4) dated and received November 13, 2009. Your submission of November 13, 2009, also constitutes a complete response to our October 16, 2009, action letter for supplemental application S-013. -
Onglyza (Saxagliptin) Uses, Dosage, Side Effects - Drugs.Com 04/07/2015 Insulin Pump – Tubeless Feel the Freedom for Yourself! Learn About the Omnipod®
Onglyza (saxagliptin) Uses, Dosage, Side Effects - Drugs.com 04/07/2015 Insulin Pump – Tubeless Feel The Freedom For Yourself! Learn About The OmniPod®. Browse all medications A B C D E F G H I J K L M N O P Q R S T U V W X Y Z Advanced Search Phonetic Search Drugs A-Z Pill Identifier Interactions Checker News Health Professionals Q & A Mednotes Apps Home → Conditions → Diabetes, Type 2 → Onglyza Print Share Sign In or Register Onglyza Related Information Availability Pregnancy Category Generic Name: saxagliptin (SAX a GLIP tin) Prescription only No proven risk in Brand Names: Onglyza humans CSA Schedule Not a controlled drug Approval History Drug history at FDA Overview Side Effects Dosage Interactions For Professionals More Reviews Average User Rating Insulin Pump – Tubeless Feel The Freedom For Yourself! Learn About The 9 User Reviews 7.6 Rate it! OmniPod® Drug Class What is Onglyza? Dipeptidyl peptidase 4 inhibitors Onglyza (saxagliptin) is an oral diabetes medicine that helps control blood sugar levels . It works by regulating Related Drugs the levels of insulin your body produces after Diabetes, Type 2 eating. metformin Onglyza is for people with type 2 diabetes. It is insulin aspart Januvia sometimes used in combination with other glipizide diabetes medications, but is not for treating glimepiride type 1 diabetes . Lantus Onglyza may also be used for purposes not Invokana listed in this medication guide. Victoza glyburide Levemir Humalog Actos Important information Janumet You should not use Onglyza if you are in a state of diabetic ketoacidosis (call your doctor for Glucophage treatment with insulin). -

Komboglyze, INN-Saxagliptin, Metformin
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Komboglyze 2.5 mg/850 mg film-coated tablets Komboglyze 2.5 mg/1,000 mg film-coated tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Komboglyze 2.5 mg/850 mg film-coated tablets Each tablet contains 2.5 mg of saxagliptin (as hydrochloride) and 850 mg of metformin hydrochloride. Komboglyze 2.5 mg/1,000 mg film-coated tablets Each tablet contains 2.5 mg of saxagliptin (as hydrochloride) and 1,000 mg of metformin hydrochloride. For the full list of excipients, see section 6.1. 3. PHARMACEUTICAL FORM Film-coated tablet (tablet). Komboglyze 2.5 mg/850 mg film-coated tablets Light brown to brown, biconvex, round, film-coated tablets, with “2.5/850” printed on one side and “4246” printed on the other side, in blue ink. Komboglyze 2.5 mg/1,000 mg film-coated tablets Pale yellow to light yellow, biconvex, oval shaped, film-coated tablets, with “2.5/1000” printed on one side and “4247” printed on the other side, in blue ink. 4. CLINICAL PARTICULARS 4.1 Therapeutic indications Komboglyze is indicated in adults with type 2 diabetes mellitus as an adjunct to diet and exercise to improve glycaemic control: in patients inadequately controlled on their maximally tolerated dose of metformin alone in combination with other medicinal products for the treatment of diabetes, including insulin, in patients inadequately controlled with metformin and these medicinal products (see sections 4.4, 4.5 and 5.1 for available data on different combinations) in patients already being treated with the combination of saxagliptin and metformin as separate tablets. -

Pharmacokinetics of Omarigliptin, a Once-Weekly Dipeptidyl Peptidase-4 Inhibitor
Available online a t www.derpharmachemica.com ISSN 0975-413X Der Pharma Chemica, 2016, 8(12):292-295 CODEN (USA): PCHHAX (http://derpharmachemica.com/archive.html) Mini-review: Pharmacokinetics of Omarigliptin, a Once-weekly Dipeptidyl Peptidase-4 Inhibitor Nermeen Ashoush a,b aClinical Pharmacy and Pharmacy Practice Department, Faculty of Pharmacy, British University in Egypt, El- Sherouk city, Cairo 11837, Egypt. bHead of Health Economics Unit, Center for Drug Research and Development (CDRD), Faculty of Pharmacy, British University in Egypt, El-Sherouk city, Cairo 11837, Egypt. _____________________________________________________________________________________________ ABSTRACT The dipeptidyl peptidase-4 (DPP-4) inhibitors are novel oral hypoglycemic drugs which have been in clinical use for the past 10 years. The drugs are safe, weight neutral and widely prescribed. There are currently many gliptins approved by FDA, namely sitagliptin, vildagliptin, saxagliptin, linagliptin, alogliptin with several more in advanced stages of development. The gliptins may possess cardiovascular protective effects and their administration may promote β-cell survival; claims currently being evaluated in clinical and preclinical studies. The gliptins are an optional second-line therapy after metformin; they are generally well tolerated with low risk of hypoglycemia. The various compounds differ with respect to their pharmacokinetic properties; however, their clinical efficacy appears to be similar. The clinical differences between the various compounds -

TREATMENT of TYPE 2 DIABETES with BIPHASIC INSULIN ANALOGUES *Ali A
TREATMENT OF TYPE 2 DIABETES WITH BIPHASIC INSULIN ANALOGUES *Ali A. Rizvi Professor of Medicine, Department of Medicine and Director, Division of Endocrinology, University of South Carolina School of Medicine, Columbia, South Carolina, USA *Correspondence to [email protected] Disclosure: The author has received grant support, as principal investigator at the University of South Carolina site, from the National Institutes of Health (NIH) for the SPRINT Trial (Contract Number: HHSN268200900040C, ClinicalTrials.gov Identifier: NCT01206062). The contents of this paper do not necessarily represent the views of the NIH. Received: 29.03.16 Accepted: 09.09.16 Citation: EMJ Diabet. 2016;4[1]:74-83. ABSTRACT The majority of patients with Type 2 diabetes require insulin therapy for treating hyperglycaemia. There are several regimens available for insulin initiation and maintenance. Insulin analogues have been developed to mimic normal physiology as closely as possible. Biphasic analogues can target both fasting and postprandial hyperglycaemia, with the added advantage of being premixed and thus convenient for the patient. A practical and feasible option is to initiate insulin with one or more biphasic preparations at mealtimes, thus providing both basal and prandial coverage. Individual titration of dose and frequency of daily injections with biphasic insulin preparations has the potential for improving glycaemic control with a high degree of patient acceptance. Drawbacks include a more rigid regimen, a relative lack of flexibility, and a somewhat higher degree of glycaemic variability and hypoglycaemia when compared to multiple daily basal-bolus injections. Awareness of the advantages and limitations of biphasic insulin analogues can assist clinicians in their appropriate use for the treatment of patients with Type 2 diabetes. -
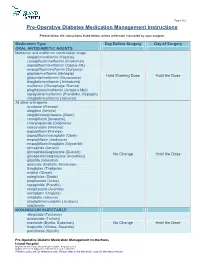
Pre-Operative Diabetes Medication Management Instructions
Page 1 of 2 Pre-Operative Diabetes Medication Management Instructions Please follow the instructions listed below unless otherwise instructed by your surgeon Medication Type Day Before Surgery Day of Surgery ORAL ANTIDIABETIC AGENTS Metformin and metformin combination drugs alogliptin/metformin (Kazano) canagliflozin/metformin (Invokamet) dapagliflozin/metformin (Xigduo XR) empagliflozin/metformin (Synjardy) glipizide/metformin (Metaglip) Hold Evening Dose Hold the Dose glyburide/metformin (Glucovance) linagliptin/metformin (Jentadueto) metformin (Glucophage, Riomet) pioglitazone/metformin (Actoplus Met) repaglidine/metformin (PrandiMet, Repaglin) sitagliptin/metformin (Janumet) All other oral agents acarbose (Precose) alogliptin (Nesina) alogliptin/pioglitazone (Oseni) canagliflozin (Invokana) chlorpropamide (Diabinese) colesevelam (Welchol) dapagliflozin (Farxiga) dapagliflozin/saxagliptin (Qtern) empagliflozin (Jardiance) empagliflozin/linagliptin (Glyxambi) glimepiride (Amaryl) glimepiride/pioglitazone (Duetact) No Change Hold the Dose glimepiride/rosiglitazone (Avandaryl) glipizide (Glucotrol) glyburide (DiaBeta, Micronase) linagliptan (Tradjenta) miglitol (Glyset) nateglinide (Starlix) pioglitazone (Actos) repaglinide (Prandin) rosiglitazone (Avandia) saxagliptin (Onglyza) sitagliptin (Januvia) sitagliptin/simvastatin (Juvisync) tolazamide NON-INSULIN INJECTABLE albiglutide (Tanzeum) dulaglutide (Trulicity) exenatide (Byetta, Bydureon) No Change Hold the Dose liraglutide (Victoza, Saxenda) pramlintide (Symlin) Pre-Operative Diabetic -

AVANDIA (Rosiglitazone Maleate Tablets), for Oral Use Ischemic Cardiovascular (CV) Events Relative to Placebo, Not Confirmed in Initial U.S
HIGHLIGHTS OF PRESCRIBING INFORMATION ----------------------- WARNINGS AND PRECAUTIONS ----------------------- These highlights do not include all the information needed to use • Fluid retention, which may exacerbate or lead to heart failure, may occur. AVANDIA safely and effectively. See full prescribing information for Combination use with insulin and use in congestive heart failure NYHA AVANDIA. Class I and II may increase risk of other cardiovascular effects. (5.1) • Meta-analysis of 52 mostly short-term trials suggested a potential risk of AVANDIA (rosiglitazone maleate tablets), for oral use ischemic cardiovascular (CV) events relative to placebo, not confirmed in Initial U.S. Approval: 1999 a long-term CV outcome trial versus metformin or sulfonylurea. (5.2) • Dose-related edema (5.3) and weight gain (5.4) may occur. WARNING: CONGESTIVE HEART FAILURE • Measure liver enzymes prior to initiation and periodically thereafter. Do See full prescribing information for complete boxed warning. not initiate therapy in patients with increased baseline liver enzyme levels ● Thiazolidinediones, including rosiglitazone, cause or exacerbate (ALT >2.5X upper limit of normal). Discontinue therapy if ALT levels congestive heart failure in some patients (5.1). After initiation of remain >3X the upper limit of normal or if jaundice is observed. (5.5) AVANDIA, and after dose increases, observe patients carefully for signs • Macular edema has been reported. (5.6) and symptoms of heart failure (including excessive, rapid weight gain; • Increased incidence of bone fracture was observed in long-term trials. dyspnea; and/or edema). If these signs and symptoms develop, the heart (5.7) failure should be managed according to current standards of care. -

OSENI (Alogliptin and Pioglitazone) Tablets II May Increase Risk
HIGHLIGHTS OF PRESCRIBING INFORMATION -----------------------WARNINGS AND PRECAUTIONS--------------------- These highlights do not include all the information needed to use • Congestive heart failure: Fluid retention may occur and can OSENI safely and effectively. See full prescribing information for exacerbate or lead to congestive heart failure. Combination use OSENI. with insulin and use in congestive heart failure NYHA Class I and OSENI (alogliptin and pioglitazone) tablets II may increase risk. Monitor patients for signs and symptoms. Initial U.S. Approval: 2013 (5.1) • Acute pancreatitis: There have been postmarketing reports of WARNING: CONGESTIVE HEART FAILURE acute pancreatitis. If pancreatitis is suspected, promptly See full prescribing information for complete boxed warning discontinue OSENI. (5.2) • Thiazolidinediones, including pioglitazone, cause or • Hypersensitivity: There have been postmarketing reports of exacerbate congestive heart failure in some patients. (5.1) serious hypersensitivity reactions in patients treated with alogliptin • After initiation of OSENI and after dose increases, monitor such as anaphylaxis, angioedema and severe cutaneous adverse patients carefully for signs and symptoms of heart failure reactions. In such cases, promptly discontinue OSENI, assess for (e.g., excessive, rapid weight gain, dyspnea and/or other potential causes, institute appropriate monitoring and edema). If heart failure develops, it should be managed treatment and initiate alternative treatment for diabetes. (5.3) according to current standards of care and • Hepatic effects: Postmarketing reports of hepatic failure, discontinuation or dose reduction of pioglitazone in OSENI sometimes fatal. Causality cannot be excluded. If liver injury is must be considered. detected, promptly interrupt OSENI and assess patient for • OSENI is not recommended in patients with symptomatic probable cause, then treat cause if possible, to resolution or heart failure. -

Reference ID: 3912436 FULL PRESCRIBING INFORMATION
HIGHLIGHTS OF PRESCRIBING INFORMATION Hypoglycemia: In add-on to sulfonylurea, add-on to insulin, and add-on These highlights do not include all the information needed to use to metformin plus sulfonylurea trials, confirmed hypoglycemia was ONGLYZA safely and effectively. See full prescribing information for more common in patients treated with ONGLYZA compared to placebo. ONGLYZA. When used with an insulin secretagogue (e.g., sulfonylurea) or insulin, a lower dose of insulin secretagogue or insulin may be required to ONGLYZA (saxagliptin) tablets, for oral use minimize the risk of hypoglycemia. (5.3, 6.1) Initial U.S. Approval: 2009 Hypersensitivity-Related Events (e.g., urticaria, facial edema): More common in patients treated with ONGLYZA than in patients treated -------------------------- RECENT MAJOR CHANGES -------------------------- with placebo; and postmarketing reports of serious hypersensitivity Warnings and Precautions reactions such as anaphylaxis, angioedema, and exfoliative skin Pancreatitis (5.1) 4/2016 conditions. Promptly discontinue ONGLYZA, assess for other potential Heart Failure (5.2) 4/2016 causes, institute appropriate monitoring and treatment, and initiate alternative treatment for diabetes. (5.4, 6.1, 6.2) --------------------------- INDICATIONS AND USAGE -------------------------- Arthralgia: Severe and disabling arthralgia has been reported in patients ONGLYZA is a dipeptidyl peptidase-4 (DPP4) inhibitor indicated as an taking DPP4 inhibitors. Consider as a possible cause for severe joint adjunct to diet and exercise to improve glycemic control in adults with type 2 pain and discontinue drug if appropriate. (5.5) diabetes mellitus. (1.1, 14) Macrovascular Outcomes: There have been no clinical studies Limitation of use: establishing conclusive evidence of macrovascular risk reduction with Not used for the treatment of type 1 diabetes mellitus or diabetic ONGLYZA or any other antidiabetic drug.