Analysis of Water Quality Using Physico-Chemical Parameters from Various Dams of Amaravati District, Maharashtra, India
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

District Taluka Center Name Contact Person Address Phone No Mobile No
District Taluka Center Name Contact Person Address Phone No Mobile No Mhosba Gate , Karjat Tal Karjat Dist AHMEDNAGAR KARJAT Vijay Computer Education Satish Sapkal 9421557122 9421557122 Ahmednagar 7285, URBAN BANK ROAD, AHMEDNAGAR NAGAR Anukul Computers Sunita Londhe 0241-2341070 9970415929 AHMEDNAGAR 414 001. Satyam Computer Behind Idea Offcie Miri AHMEDNAGAR SHEVGAON Satyam Computers Sandeep Jadhav 9881081075 9270967055 Road (College Road) Shevgaon Behind Khedkar Hospital, Pathardi AHMEDNAGAR PATHARDI Dot com computers Kishor Karad 02428-221101 9850351356 Pincode 414102 Gayatri computer OPP.SBI ,PARNER-SUPA ROAD,AT/POST- 02488-221177 AHMEDNAGAR PARNER Indrajit Deshmukh 9404042045 institute PARNER,TAL-PARNER, DIST-AHMEDNAGR /221277/9922007702 Shop no.8, Orange corner, college road AHMEDNAGAR SANGAMNER Dhananjay computer Swapnil Waghchaure Sangamner, Dist- 02425-220704 9850528920 Ahmednagar. Pin- 422605 Near S.T. Stand,4,First Floor Nagarpalika Shopping Center,New Nagar Road, 02425-226981/82 AHMEDNAGAR SANGAMNER Shubham Computers Yogesh Bhagwat 9822069547 Sangamner, Tal. Sangamner, Dist /7588025925 Ahmednagar Opposite OLD Nagarpalika AHMEDNAGAR KOPARGAON Cybernet Systems Shrikant Joshi 02423-222366 / 223566 9763715766 Building,Kopargaon – 423601 Near Bus Stand, Behind Hotel Prashant, AHMEDNAGAR AKOLE Media Infotech Sudhir Fargade 02424-222200 7387112323 Akole, Tal Akole Dist Ahmadnagar K V Road ,Near Anupam photo studio W 02422-226933 / AHMEDNAGAR SHRIRAMPUR Manik Computers Sachin SONI 9763715750 NO 6 ,Shrirampur 9850031828 HI-TECH Computer -
0001S07 Prashant M.Nijasure F 3/302 Rutu Enclave,Opp.Muchal
Effective Membership ID Name Address Contact Numbers from Expiry F 3/302 Rutu MH- Prashant Enclave,Opp.Muchala 9320089329 12/8/2006 12/7/2007 0001S07 M.Nijasure Polytechnic, Ghodbunder Road, Thane (W) 400607 F 3/302 Rutu MH- Enclave,Opp.Muchala Jilpa P.Nijasure 98210 89329 8/12/2006 8/11/2007 0002S07 Polytechnic, Ghodbunder Road, Thane (W) 400607 MH- C-406, Everest Apts., Church Vianney Castelino 9821133029 8/1/2006 7/30/2011 0003C11 Road-Marol, Mumbai MH- 6, Nishant Apts., Nagraj Colony, Kiran Kulkarni +91-0233-2302125/2303460 8/2/2006 8/1/2007 0004S07 Vishrambag, Sangli, 416415 MH- Ravala P.O. Satnoor, Warud, Vasant Futane 07229 238171 / 072143 2871 7/15/2006 7/14/2007 0005S07 Amravati, 444907 MH MH- Jadhav Prakash Bhood B.O., Khanapur Taluk, 02347-249672 8/2/2006 8/1/2007 0006S07 Dhondiram Sangli District, 415309 MH- Rajaram Tukaram Vadiye Raibag B.O., Kadegaon 8/2/2006 8/1/2007 0007S07 Kumbhar Taluk, Sangli District, 415305 Hanamant Village, Vadiye Raibag MH- Popat Subhana B.O., Kadegaon Taluk, Sangli 8/2/2006 8/1/2007 0008S07 Mandale District, 415305 Hanumant Village, Vadiye Raibag MH- Sharad Raghunath B.O., Kadegaon Taluk, Sangli 8/2/2006 8/1/2007 0009S07 Pisal District, 415305 MH- Omkar Mukund Devrashtra S.O., Palus Taluk, 8/2/2006 8/1/2007 0010S07 Vartak Sangli District, 415303 MH MH- Suhas Prabhakar Audumbar B.O., Tasgaon Taluk, 02346-230908, 09960195262 12/11/2007 12/9/2008 0011S07 Patil Sangli District 416303 MH- Vinod Vidyadhar Devrashtra S.O., Palus Taluk, 8/2/2006 8/1/2007 0012S07 Gowande Sangli District, 415303 MH MH- Shishir Madhav Devrashtra S.O., Palus Taluk, 8/2/2006 8/1/2007 0013S07 Govande Sangli District, 415303 MH Patel Pad, Dahanu Road S.O., MH- Mohammed Shahid Dahanu Taluk, Thane District, 11/24/2005 11/23/2006 0014S07 401602 3/4, 1st floor, Sarda Circle, MH- Yash W. -

University Grants Commission Socio and Economic Study and Critical Analysis of the Causes and Factors Leading to the Suicides Of
University Grants Commission (W.R.O) Pune SUMMARY - Minor Research Project - * Subject * Socio And Economic Study and critical analysis of the Causes and Factors Leading to the Suicides of Farmers in Amravati District Name of Teacher – Kumar Bobade. File NO. 23 – 007/6 (WRO) Dated 03/02/07. Amount Sanction Rs. 35000/- Shri Shivaji College, Amravati. MINOR REASERCH PROJECT Summary “Socio And Economic Study and Critical analysis of the Causes and Factors Leading to the Suicides of Farmers in Amravati District” Chapter I - Introduction India is agriculture based country. Near about 80% population means more than 2/3 nation belongs to villages. Agriculture and agriculture based industries are only meant for bread and butter for these people. It is a well-known fact that agriculture is the backbone of economy of this country. Father of nation’s Mahatma Gandhi was well familiar with this fact and that is why agriculture and related industries were emphasized by him. The Government since independence has given the importance to the agriculture and agriculture based industries and various agriculture development schemes have been launched. In five year development plan Agriculture is the prominent factor. Green revolution was planned and succeeded to develop agriculture and self- independence in food grains to make it prosper and self-independent. It was the beginning for adopting new technologies, new methods and inventions in agriculture. Agriculture universities were established. But it is a question that did they fulfill the needs and useful betterment of farmers? Can we say that the farmers in this country are self-developed, mentally capable and economically stabled? Overall answer is Negative. -

Pre-Feasibility Report Proposed Ajansara Barrage Project for Lift
PRE-FEASIBILITY REPORT PROPOSED AJANSARA BARRAGE PROJECT M/s. Vidharbha FOR LIFT IRRIGATION CAPACITY:TOTAL 30004 CCA Irrigation Development at Village Ajansara, Taluka Hinganghat, District Wardha, Maharashtra Corporation (VIDC) STUDY PERIOD PROPOSED AJANSARA BARRAGE PROJECT FOR LIFT IRRIGATION FOR TOTAL 30004 CCA AND 24000 ICA AT VILLAGE AJANSARA, TALUKA HINGANGHAT, DISTRICT WARDHA, MS INDEX BY M/S. VIDHARBHA IRRIGATION DEVELOPMENT CORPORATION (VIDC) INDEX Sr. No. Particular Page No. 1 Executive Summary 1 2 INTRODUCTION OF THE PROJECT/ BACKGROUND 6 INFORMATION 2.1 Identification of project 6 2.2 Brief History of nature of the project 7 2.3 Need for the project and its importance to the country and 7 region 2.4 Benefit of Project 9 3 PROJECT DESCRIPTION 10 3.1 Type of project including interlinked and interdependent 10 projects, if any 3.2 Regulatory Frame Work 10 3.3 Location (map showing general location, specific location, 11 and project boundary & project site layout) with coordinates 3.4 Details of alternate sites considered and the basis of 21 selecting the proposed site, particularly the environmental considerations gone into should be highlighted 3.5 Size or magnitude of operation 21 3.6 Project description with process details (a schematic 21 diagram/ flow chart showing the project layout, components of the project etc. 3.6.1 Design Feature of Head Work 21 3.6.2 Rolled Filled Earth Dam 22 3.6.3 Barrage 23 3.6.4 Design of Barrage 23 3.6.5 Foundation of Barrage 23 4 IRRIGATION PLANNING 24 4.1 Existing and Proposed Facilities in Command Area 24 4.2 Existing and Proposed Cropping Patterns 24 4.3 Soil Survey 24 4.4 Evaporation Losses 25 5 SURVEY AND INVESTIGATION 25 5.1 Topographical Survey & Investigation 25 5.2 Survey for Barrage 25 5.3 Submergence Survey 25 5.4 Canal and Command Area Survey 25 5.5 Survey for Construction Material 25 5.6 Geotechnical Investigation 26 6 PROJECT HYDROLOGY 26 6.1 General Climate and Hydrology 26 6.2 Hydrological Data 27 6.2.1 Catchment Area 27 SMS Envocare Ltd. -

Physiography and Spatial Distribution of Settlements of Tribal Population in Warud Tahsil in Amravati District
PHYSIOGRAPHY AND SPATIAL DISTRIBUTION OF SETTLEMENTS OF TRIBAL POPULATION IN WARUD TAHSIL IN AMRAVATI DISTRICT DR. OMPRAKASH B. MUNDE Head, Dept. of Geography, Narayanrao Rana Mahavidyalaya Badnera Railway (MS) INDIA Relief features of any region are impact on the distribution of population as well as settlements of that region. Tribal population is generally situated in hilly region but now days some of them are well educated and settled in urban region. The height of the region is positively impact on the distribution of settlements and population of tribal community. This paper reveals the analysis of distribution of settlements and population of tribal community in Warud tahsil of Amravati district. Key Words Physiography, tribal population, distribution, settlement INTRODUCTION Warud tahsil is the part of Amravati district and near about 15% population of this tahsil is tribal and out of them more than 85% tribal population situated in rural region. Relief of the tahsil is impact on the location of tribal population. Present paper is based on the distribution and location of settlement and population of tribal community according to the height in Warud tahsil of Amravati district. Objectives The main objectives of the present research work as follows, 1) To explain the height of the study region. DR. OMPRAKASH B. MUNDE 1P a g e 2) To analysis the distribution and density of Scheduled Tribe population according to height of the region. 3) To point out the locations of settlements of tribal population according to the size of their population. 4) To calculate the relationship between physiography and distribution of ST population and their settlements. -

A Detailed District Survey Report of Amravati
DSR- AMRAVATI A Detailed District Survey Report of Amravati Collector office; Amravati. 1 DSR- AMRAVATI Index Preface 1. Introduction 2. Overview of mining activity in the district 3. List of the Mining Leases in the district 4. Details of Royalty or Revenue received in last three years 5. Details of Production of sand or minor mineral in last three years 6. Deposition of sediments in the rivers of the district 7. General profile of the district 8. Land utilization pattern in the district 9. Physiography of the district 10. Rainfall 11. Geology and Mineral wealth 12. Major and Minor Rivers of the District 13. General recommendation 2 DSR- AMRAVATI Preface In Compliance to the Notification Issued by the Ministry Of Environment, Forest and Climate change Dated 15.01.2016, the preparation of District survey report of River bed mining and other minor minerals is in accordance appendix 10 of the notification. It is also mentioned here that the procedure of preparation of District Survey Report is as per notification guidelines. Every efforts have been made to cover sand mining locations, area & overview of mining activity in the district with all its relevant features pertaining to geology & mineral wealth in replenish able and non- replenish able areas of rivers, stream and other sand sources. This report will be a model and guiding document which is a compendium of available mineral resources, geographical set up, environmental and ecological set up of the district and is based on data of various departments, published reports, and websites. The data may vary due to floods, heavy rains and other natural calamities. -

Model Agriculture Contingency Plan (Rainfed) District: Amravati State
Model Agriculture Contingency Plan (Rainfed) District: Amravati State: Maharashtra 1.0 District Agriculture profile 1.1 Agro-Climatic/Ecological Zone Agro Ecological Sub Region (ICAR) Western Maharashtra Plateau, hot moist semi-arid eco- sub region (6.3) Agro-Climatic Region (Planning Commission) Western Plateau and Hills Region (IX) Agro Climatic Zone (NARP) Central Maharashtra Plateau Zone (MH-7) List all the districts or part thereof falling under the NARP Amravati, Akola, Buldhana, Washim Zone Geographic coordinates of district headquarter Latitude Longitude Altitude 20° 55' 53.82” N 77° 45' 32.57” E 374 m above MSL Name and address of the concerned ZRS/ ZARS/ RARS/ Regional Research Center (Dr. PDKV), Morshi Road, Amravati-444603 RRS/ RRTTS Mention the KVK located in the district with full address KVK, Durgapur, Tq. Badnera Distt. Amravati- 444701 KVK, Ghatkhed, Tq. Chandur Rly. Distt. Amravati “Chirantan” Madhuban Colony,Camp,Amravati-444602 Name and address of the nearest Agromet Field Unit for AMFU Station, Akola, Maharashtra agro-advisories in the Zone 1.2 Rainfall Normal Rainy Normal Onset Normal Cessation Normal RF(mm) days (number) SW monsoon (June-September): 775.2 40 2nd week of June 1st week of October NE Monsoon(October-December): 69.6 4 - - Winter (January- February) 29.4 3 - - Summer (March-May) 12.2 1 - - Annual 886.4 48 - - Source: IMD 1.3 Land use Geographical Cultivabl Forest Land under Permanent Cultivable Land under Barren & Current Other pattern of the Area e area area non pastures waste land miscellane uncultivable fallows fallows district agricultural ous tree land use crops & groves Area (‘000 ha) 1304 766 321 16 29 20 8 28 19 97 Source: DACNET 2005-06 1. -
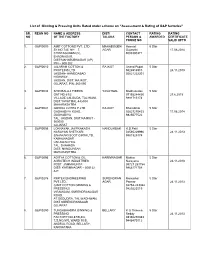
List of Ginning & Pressing Units Rated Under Scheme on “Assessment
List of Ginning & Pressing Units Rated under scheme on “Assessment & Rating of G&P factories” SR. REGN NO NAME & ADDRESS DIST/ CONTACT RATING RATING NO OF THE FACTORY TALUKA PERSON & AWARDED CERTIFICATE PHONE NO VALID UPTO 1. G&P/0009 AMIT COTTONS PVT. LTD MAHABOOBN Hemant 5 Star SY.NO.745, NH – 7, AGAR Gujarathi 17.08.2014 CHINTAGUDEM (V), 9000300371 EHADNAGAR, DIST:MAHABUBNAGAR (AP) PIN – 509 202 2. G&P/0010 JALARAM COTTON & RAJKOT Anand Popat 5 Star PROTEINS LTD 9426914910 24.11.2013 JASDAN- AHMEDABAD 02821222201 HIGHWAY, JASDAN, DIST: RAJKOT, GUJARAT, PIN: 360 050 3. G&P/0034 SHRI BALAJI FIBERS YAVATMAL Madhusudan 5 Star GAT NO:61/2 07153244430 27.6.2015 VILLAGE LALGUDA, TAL:WANI, 9881715174 DIST:YAVATMAL-445304 MAHARASHTRA 4. G&P/0041 GIRIRAJ COTEX P.LTD RAJKOT Bharatbhai 5 Star GADHADIYA ROAD, 02827270453 17.08.2014 GADHADIYA 9825077522 TAL: JASDAN, DIST;RAJKOT - 360050 GUJARAT 5. G&P/0056 LOKNAYAK JAYPRAKASH NANDURBAR R.D.Patil 5 Star NARAYAN SHETKARI 02565229996 24.11.2013 SAHAKARI SOOT GIRNI LTD, 9881925174 KAMALNAGAR UNTAWAD HOL TAL. SHAHADA DIST: NANDURBAR MAHARASHTRA 6. G&P/0096 ADITYA COTTON & OIL KARIMNAGAR Mukka 5 Star AGROTECH INDUSTRIES Narayana 24.11.2013 POST: JAMMIKUNTA 08727 253754 DIST: KARIMNAGAR – 505122 9866171754 A.P. 7. G&P/027 6 RIMTEX ENGINEERING SURENDRAN Manubhai 5 Star PVT.LTD., AGAR Parmar 24.11.2013 (UNIT COTTON GINNING & 02752-243322 PRESSING) 9825223519 VIRAMGAM, SURENDRANAGAR ROAD, AT.DEDUDRA, TAL.WADHWAN, DIST SURENDRANAGAR GUJARAT 8. G&P/0290 TUNGABHADRA GINNING & BELLARY K G Thimma 5 Star PRESSING Reddy 24.11.2013 FACTORY,NO.87/B,3/4, 08392250383 T.S.NO.970, WARD 10 B, 9448470112 ANDRAL ROAD, BELLARY, KARNATAKA 9. -

1 Wardha Arvi Grampanchayat - Amol Hinge 9823688927 '10504401753379848754 20.683139 78.747724 Wardha
ग्रामपंचायत/महानगर झोन/वा셍 ड क्र जि쥍हा ताऱुका पालऱका/नगरपररषद/ कᴂ द्र चाऱकाच े नाव मोबाईऱ क्र CSC ID आपऱे सरकार सेवा कᴂ द्राचा प配ता अऺांश रेखांश (फ啍त शहरी नगरपंचायत कᴂ द्राकरीता) Behind Grampanchayat Madana Dist. 1 Wardha Arvi Grampanchayat - Amol Hinge 9823688927 '10504401753379848754 20.683139 78.747724 Wardha 2 Wardha Arvi Nagarparishad Ward Suhas V Thakre 9545217092 10504401780269400035 Near Bank Of India Arvi Wardha 20.756533 78.61302 Maha E Seva Kendra,Shirish Digital Studio, 3 Wardha Arvi Grampanchayat - Ashish Tarale 9923275412 10504401753377748650 20.935586 78.46039 Arvi Road, Morangana Taluka Arvi 4 Wardha Arvi Grampanchayat - Kishor kalpande 9823923473 13504401753376945477 Borgaon Main Road 442201 21.273125 78.224186 Maha E seva Kendra, Grampanhayat 5 Wardha Arvi Grampanchayat - Sunanda P.Dole 9890958289 10504401753367148662 21.273125 78.224186 Road, Pachod, Taluka Arvi 6 Wardha Arvi Grampanchayat - Devidas Gujar 8600340280 13504401753382545318 12, Dhanodi (Bha) Ward No 2 442201 20.884414 78.28559 7 Wardha Arvi Grampanchayat - Sarika Gharde 9890388092 13504401753387148192 AT PULGAON WARDHA ROD 442201 20.7730497 78.355777 8 Wardha Arvi Grampanchayat - Nitin Chandanmathe 9890388092 31504401980269648846 AT PULGAON WARDHA ROD 442201 20.9625635 78.236578 at virul near kirana shop main road virul 9 Wardha Arvi Grampanchayat - Prajay Hegde 9970916890 13504401753385245384 20.804609 78.351154 442201 10 Wardha Arvi Nagarparishad Ward Rashmi Gajanan Kohad 9970651759 '10504401780269454509 Bachlor Road taluka Arvi Dist Wardha 20.992601 -

Study of Ground Water Occurrence and Future Planning in Urad Village, Warud Taluka, Amravati District, Maharashtra
International Journal of Science, Environment and Technology, Vol. 1, No 3, 2012, 205 - 216 STUDY OF GROUND WATER OCCURRENCE AND FUTURE PLANNING IN URAD VILLAGE, WARUD TALUKA, AMRAVATI DISTRICT, MAHARASHTRA Manish S. Deshmukh 1 and Khadri, S.F.R 2 1Department of Geology, Sant Gadge Baba Amravati University, Amravati (M.S.) 2Professor & Head, Department of Geology, Sant Gadge Baba Amravati University, Amravati (M.S.) E-mail: [email protected] Abstract: Village Urad lies in Warud tahsil of Amravati district, Maharashtra and is located due North-East of Amravati city at a distance of about 104 km and falls in the watershed WR-1.This area covered under dark watershed zone declared by state ground water board. During this study 100% well inventory of the existing wells, in urad village has been carried out to delineate the unconfined aquifer on the basis of detailed and comprehensive hydrogeological survey. During this study basemap of 1: 10,000 is used for field survey. There are 363 wells existing in the village. Out of them 359 wells are irrigation dug wells and 4 dug wells and one bore well fitted with hand pump are used for drinking water supply purpose. In all 287 wells are fitted with electric pump. During the well inventory, summer and winter static water levels, lithology, aquifer and other related information has been collected. The wells are connected with respect to mean sea level by leveling. Reduced level( RL) values of pre monsoon and post monsoon water level were computed and water table contour maps of village area are prepared. -

District Survey Report for Sand Mining Or River Bed Mining
DRAFT DSR REPORT DISTRICT SURVEY REPORT FOR SAND MINING OR RIVER BED MINING Prepared Under A] Appendix –X Of MoEFCC, GoI. Notification S.O. 141(E) Dated 15.1.2016 B] Sustainable Sand Mining Guidelines C] MoEFCC, GoI. Notification S.O. 3611(E) Dated 25.07.2018 AMRAVATI - 2019-20 MAHARASHTRA PREFACE The Ministry of Environment, Forests & Climate Change (MoEF&CC), Government of India, made Environmental Clearance (EC) for mining of minerals mandatory through its Notification of 27th January, 1994 under the provisions of Environment Protection Act, 1986. Keeping in view the experience gained in environmental clearance process over a period of one decade, the MoEF&CC came out with Environmental Impact Notification, SO 1533 (E), dated 14th September 2006. Now again Ministry of Environment, Forests & Climate Change (MoEF&CC), Government of India amended the notification S.O. th 141(E) Dated 15TH January, 2016 vide S.O. 3611(E) Dated 25 July, 2018. It has been made mandatory to obtain environmental clearance for different kinds of development projects as listed in Appendix-X of the Notification. Further, In pursuance to the order of Hon’ble Supreme Court dated the 27th February, 2012 in I.A. No.12- 13 of 2011 in Special Leave Petition (C) No.19628-19629 of 2009, in the matter of Deepak Kumar etc. Vs. State of Haryana and Others etc., prior environmental clearance has now become mandatory for mining of minor minerals irrespective of the area of mining lease; And also in view of the Hon’ble National Green Tribunal, order dated the 13TH January, -

Mechanism of Interlinking of WARDHA Rivers Using ERDAS Model
ISSN(Online) : 2319-8753 ISSN (Print) : 2347-6710 International Journal of Innovative Research in Science, Engineering and Technology (An ISO 3297: 2007 Certified Organization) Website: www.ijirset.com Vol. 6, Issue 3, March 2017 Mechanism of Interlinking of WARDHA Rivers Using ERDAS Model Vaishnavi Vinod Borkar, Prof. Deepali Kulkarni, Prof. R. H. Jadhav M.Tech. Student, Department of Civil Engineering, Bharti Vidyapeeth Deemed University (BVDU), College of Engineering, Pune (COEP), India Assistant Professor, Department of Civil Engineering, Bharti Vidyapeeth Deemed University (BVDU), College of Engineering Pune (COEP), India Associate Professor, Department of Civil Engineering, Bharti Vidyapeeth Deemed University (BVDU), College of Engineering Pune (COEP), India ABSTRACT: Water has been perceived as fundamental and vital characteristic assets for general improvement of a country. Be that as it may, the accessibility of water does not coordinate with the request in term of amount and quality in time and space. To satisfy the necessity of water for water system and water supply, humanity has been misusing the surface or subsurface assets locally since long. The orange developing zone, prominently known as "California of Maharashtra" and the Cotton developing territory , prevalently known as Manchester of India are arranged in this locale. In any case, now a days by over abuse of surface and ground water, the supposed Cotton developing zone is drawing nearer towards the leave and also because of absence of one watering to the cotton, declination in product yield which causes the enormous suicide instances of the cultivators. The shortage of water in Akola, Amravati, Washim, Buldhana, Yeotmal city in 2005 wanted to convey water from upper Wardha Project.