Fetal Hydrops – a Review and a Clinical Approach to Identifying the Cause
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Meconium Peritonitis
ISSN: 2377-9004 Agrawal et al. Obstet Gynecol Cases Rev 2020, 7:180 DOI: 10.23937/2377-9004/1410180 Volume 6 | Issue 5 Obstetrics and Open Access Gynaecology Cases - Reviews CASE REPORT Meconium Peritonitis: In Utero Diagnosis of a Rare Clinical Entity and Postnatal Outcome Sarita Agrawal, MD, FICOG, FIAMS, FCGP1, Arpana Verma, MS2*, Sarita Rajbhar, MS3, Pushpawati Thakur, MD4, Loukya Kodumuri, MBBS5 and Swati Kumari, MS, FNB6 1Professor and Head of Department, Department of Obstetrics and Gynaecology, All India Institute of Medical Sciences, Raipur, Chhattisgarh, India 2Senior Resident, Department of Obstetrics and Gynaecology, All India Institute of Medical Sciences, Raipur, Chhattisgarh, India 3Assistant Professor, Department of Obstetrics and Gynaecology, All India Institute of Medical Sciences, Raipur, Chhattisgarh, India 4 Check for Associate Professor, Department of Obstetrics and Gynaecology, All India Institute of Medical Sciences, updates Raipur, Chhattisgarh, India 5Post Graduate Student, Department of Obstetrics and Gynaecology, All India Institute of Medical Sciences, Raipur, Chhattisgarh, India 6Sonologist and Fetal Medicine Expert, Shivam Fetomed and Spine Centre, Raipur, Chhattisgarh, India *Corresponding author: Arpana Verma, MS, Senior Resident, Department of Obstetrics and Gynaecology, All India Institute of Medical Sciences, V.V-18, Parthivi Province, Near Salasar Greens, Sarona, Raipur, Chhattisgarh - 492099, India, Tel: 9741412716; 6260334337 Abstract done for immune and non-immune hydrops and was found to be negative. One week later, a repeat ultrasound was done Objective: To present an unusual case of meconium peri- which showed moderate fetal ascites with few areas of calci- tonitis diagnosed during prenatal period and its postnatal fication in the bowel loops and prominent inferior vena cava, outcome. -
CASE REPORT CASE CASE R Imaging Findings of Meconium
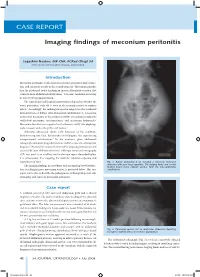
CASE REPORT CASE REPORT Imaging findings of meconium peritonitis Logeshini Naidoo, MB ChB, FCRad (Diag) SA Helen Joseph and Coronation Hospitals, Johannesburg Introduction Meconium peritonitis results from intrauterine gastrointestinal perfora- tion, and can occur as early as the second trimester.1 Meconium extrudes into the peritoneal cavity, inciting an intense fibroplastic reaction that results in intra-abdominal calcifications.2 It is a rare condition occurring in 1 in 35 000 pregnant women.3 The clinical and radiological manifestations depend on whether the bowel perforation seals off in utero in the neonatal period or remains patent.3 Accordingly, the radiological spectra range from the incidental demonstration of diffuse intra-abdominal calcifications to meconium ascites (free meconium in the peritoneal cavity), meconium pseudocysts (walled-off meconium concentrations), and meconium hydrocoeles. Meconium has also been reported in the thoracic cavity (via diaphrag- matic hernias) and in the pelvic soft tissues.4 Antenatal ultrasound allows early detection of the condition, demonstrating free fluid, hydrocoeles and echogenic foci representing intraperitoneal calcifications.5 In the newborn, plain abdominal radiographs demonstrating calcifications and/or ascites are sufficient for diagnosis.1 Postnatal ultrasound is reserved for atypical presentations and can exclude intra-abdominal masses.1 Although computed tomography (CT) was used as an ancillary tool in the case report described below, it is unnecessary, thus negating the need for radiation exposure and expenditure of time. Fig. 1. Supine abdominal X-ray revealing a massively distended abdomen with poor lung capacities. The bulging flanks and central The imaging findings in a newborn with an ongoing bowel perfora- floating bowel loops indicate ascites. -

Fetal Meconium Peritonitis Associated with Prenatal Methamphetamine Exposure
W.J. Yang, et al ■ SHORT COMMUNICATION ■ FETAL MECONIUM PERITONITIS ASSOCIATED WITH PRENATAL METHAMPHETAMINE EXPOSURE Wen-Jui Yang1, Chie-Pein Chen1,2*, Chen-Yu Chen1, Kuo-Gon Wang1, Tsung-Hsien Su1,2 1Department of Obstetrics and Gynecology, Mackay Memorial Hospital, and 2Mackay Medicine, Nursing and Management College, Taipei, Taiwan. SUMMARY Objective: In roughly 50% of all patients with meconium peritonitis, there is no evidence of primary obstruction of the bowel. We report a case of maternal methamphetamine and heroin abuse complicated by fetal meconium pseudocyst without a definite intestinal obstructive lesion. We discuss the correlation between the presence of meconium peritonitis and prenatal exposure to methamphetamine. Case Report: A 19-year-old, gravida 2, para 0, abortus 1, woman had abused illegal drugs, including methamphetamine and intravenous heroin. Suffering from withdrawal symptoms, she was taken to a shelter where she was diagnosed with a pregnancy of 26 weeks’ gestation. The patient underwent cesarean section at 34 weeks due to preterm labor and fetal malpresentation. A boy weighing 2,474 g was born, but had to be intubated and admitted to the intensive care unit because of a distended abdomen and respiratory distress. A laparotomy performed on the second day of life revealed a large calcified pseudocyst associated with two perforations of the distal jejunum. A segmental resection of the jejunum with primary anastomosis was performed. The infant recovered well after the operation and was discharged 65 days after birth. Conclusion: Since methamphetamine is a powerful _-adrenergic stimulant and induces the release of catecholamines from adrenergic synapses, it can cause powerful vessel constriction. -

Long-Term Outcome After Neonatal Meconium Obstruction
Long-term Outcome After Neonatal Meconium Obstruction Julie R. Fuchs, MD, and Jacob C. Langer, MD ABSTRACT. Objective. It is unclear whether children meconium ileus and those undergoing resection or enter- with cystic fibrosis (CF) who present with neonatal ostomy. Patients with meconium obstruction who do not meconium ileus have a different long-term outcome from have CF have an excellent long-term prognosis. This those presenting later in childhood with pulmonary com- information will be useful in counseling the families of plications or failure to thrive. We examined a cohort of infants presenting with neonatal meconium obstruction. patients with meconium ileus, and compared their long- Pediatrics 1998;101(4). URL: http://www.pediatrics.org/ term outcome with children who had CF without meco- cgi/content/full/101/4/e7; cystic fibrosis, meconium ileus, nium ileus and neonates who had meconium obstruction meconium plug syndrome. without CF (meconium plug syndrome). Study Design. Comparative study using retrospective and follow-up interview data. ABBREVIATION. CF, cystic fibrosis. Patients. Group 1 consisted of 35 surviving CF pa- tients who presented with meconium ileus between 1966 econium obstruction in the neonate is a and 1992. Two control groups were also studied: 35 age- spectrum of disease that includes meco- and sex-matched CF patients without meconium ileus 1 (group 2), and 12 infants presenting with meconium plug Mnium ileus and meconium plug syndrome. syndrome during the same time period (group 3). Meconium ileus is characterized by extremely viscid, Outcome Measures. Pulmonary, gastrointestinal, nu- protein-rich inspissated meconium causing terminal tritional, and functional status were reviewed, and sur- ileal obstruction, and accounts for approximately gical complications were recorded. -

PROBLEMS of the NEONATAL PERIOD
PROBLEMS of the NEONATAL PERIOD Susan Fisher-Owens, MD, MPH, FAAP Associate Clinical Professor of Clinical Pediatrics Associate Clinical Professor of Preventive and Restorative Dental Sciences University of California, San Francisco Zuckerberg San Francisco General Hospital UCSF Family Medicine Board Review: Improving Clinical Care Across the Lifespan San Francisco March 6, 2017 Disclosures “I have nothing to disclose” (financially) …except appreciation to Colin Partridge, MD, MPH for help with slides 2 Common Neonatal Problems Hypoglycemia Respiratory conditions Infections Polycythemia Bilirubin metabolism/neonatal jaundice Bowel obstruction Birth injuries Rashes Murmurs Feeding difficulties 3 Abbreviations CCAM—congenital cystic adenomatoid malformation CF—cystic fibrosis CMV—cytomegalovirus DFA-- Direct Fluorescent Antibody DOL—days of life ECMO—extracorporeal membrane oxygenation (“bypass”) HFOV– high-flow oxygen ventilation iNO—inhaled nitrous oxide PDA—patent ductus arteriosus4 Hypoglycemia Definition Based on lab Can check a finger stick, but confirm with central level 5 Hypoglycemia Causes Inadequate glycogenolysis cold stress, asphyxia Inadequate glycogen stores prematurity, postdates, intrauterine growth restriction (IUGR), small for gestational age (SGA) Increased glucose consumption asphyxia, sepsis Hyperinsulinism Infant of Diabetic Mother (IDM) 6 Hypoglycemia Treatment Early feeding when possible (breastfeeding, formula, oral glucose) Depending on severity of hypoglycemia and clinical findings, -

Cystic Fibrosis Discussants IVAN HARWOOD, MD; FERNANDO ROSAS, MD; DAVID K
62 im A Cystic Fibrosis Discussants IVAN HARWOOD, MD; FERNANDO ROSAS, MD; DAVID K. EDWARDS, MD; JOHN KELSO, MD; and WILLIAM L. NYHAN, MD, PhD I VAN HARWOOD, MD: * A starting point for the discussion of partial carbon dioxide pressure of 52 torr, and it was decided important topics in cystic fibrosis and its management in to insert an endotracheal tube for ventilation and to begin infants is provided by an informative case of a patient, which treatment with ribavirin. Klebsiella and Escherichia coli will be presented by Dr Rosas. From there we will discuss the were found on tracheal culture. rapidly developing advances in diagnosis and therapy. His course was stormy because of recurrent episodes of increased airway resistance and increased difficulty in ven- Case Presentation tilation, but his condition slowly improved, and the endo- Case 1 tracheal tube was removed about a week later. His weight was FERNANDO RoSAS, MD:t The mother was seen because of 2.3 kg. At 2 months of age, sufficient sweat could be collected polyhydramnios and other factors that led to the decision to for the first time for a sweat test, which was positive; the deliver the infant at 33 weeks by cesarean section. She was 30 chloride concentration was 95.7 mEq per liter and the spec- years old and the father 39. The first offive siblings died at the imen weighed 130 mg. age of 15 months of what was called intestinal infection, the Six weeks after admission, he was discharged weighing fourth was a fetal death at 20 weeks of gestation, and the fifth 2.7 kg and tolerating Pregestimil (Mead Johnson) formula died at 3 months of age of pneumonia. -

Meconium Peritonitis Due to Fetal Appendiceal
Wang et al. BMC Pediatrics (2018) 18:162 https://doi.org/10.1186/s12887-018-1133-8 CASEREPORT Open Access Meconium peritonitis due to fetal appendiceal perforation: two case reports and a brief review of the literature Yi Wang1, Yeming Wu1, Wenbin Guan2, Wenbo Yan1, Yuhua Li3, Jin Fang3 and Jun Wang1* Abstract Background: Meconium peritonitis is an infrequent congenital disease usually caused by perforation of the fetal digestive tract. Meconium peritonitis resulting from intrauterine appendiceal perforation has been rarely reported and is often overlooked during pregnancy. We herein report two cases of fetal appendiceal perforation. Case presentation: Two neonates were found to have intestinal distension and gradually increasing ascites antenatally. After birth, diagnostic abdominal punctures revealed meconium peritonitis. Urgent surgery showed both neonates had developed gangrenous appendicitis in utero. Pathological examination supported the diagnosis of fetal appendiceal perforation in both neonates, and one also had deformity of cecal duplication. In the present report, we also review the presentation, diagnosis, pathology, management, and recent literature of fetal appendiceal perforation. Conclusion: Meconium peritonitis due to fetal appendiceal perforation is extremely rare, and preoperative diagnosis is almost impossible. However, clinicians should be aware of abnormal gastrointestinal manifestations in the fetus during the antenatal examination. For neonates with severe meconium peritonitis, an early operation with careful intraoperative exploration is necessary. Keywords: Meconium peritonitis, Appendicitis, Intestinal duplication, Fetus, Surgery Background experience with special reference to the clinical presen- Meconium peritonitis (MP) is a sterile chemical periton- tation, evaluation (particularly with respect to the pre- itis that is caused by intrauterine bowel perforation and operative diagnosis and pathological diagnosis), and has low morbidity (1/30,000). -

Pulmonary Hypoplasia: Lung Weight and Radial Alveolar Count As Criteria of Diagnosis
Arch Dis Child: first published as 10.1136/adc.54.8.614 on 1 August 1979. Downloaded from Archives of Disease in Childhood, 1979, 54, 614-618 Pulmonary hypoplasia: lung weight and radial alveolar count as criteria of diagnosis S. S. ASKENAZI AND M. PERLMAN Hadassah Hospital, Jerusalem SUmmARY A working definition of pulmonary hypoplasia (PH) was established by retrospective assessment of lung growth both in recognised and hypothetical PH-associated conditions. Lung weight: body weight ratios (LW:BW) were calculated, and morphometry was determined by the radial alveolar count (RAC) (Emery and Mithal, 1960). Both parameters were reduced compared with those of normal controls in diaphragmatic hernia, anencephalus, anuric renal anomalies, chondrodystrophies, and osteogenesis inperfecta. Comparison of LW:BW ratio and RAC indicated that the RAC was the more reliable criterion of PH, LW:BW ratio of .0-012 (67% of mean nor- mal ratio) and/or RAC of < 4.1 (75 % of mean normal count) are suggested as diagnostic criteria of PH. Evidence ofPH was incidentally discovered in a number ofclinically unsuspected cases and retro- spectively clarified the clinical and radiological findings. Routine assessment of lung growth should be an essential part of the neonatal necropsy. Pulmonary hypoplasia (PH) is a poorly defined establish. There has been only one study of both copyright. condition considered to be almost invariably LW:BW ratio and morphometry in a substantial secondary to other anomalies and is usually diag- number of pathological cases (Reale and Esterly, nosed in association with them; primary or isolated 1973). PH has not been reported (Reale and Esterly, 1973). -

Can Early Surgery Improve the Outcome of Patients with Meconium
Jiang et al. BMC Pediatrics (2019) 19:473 https://doi.org/10.1186/s12887-019-1844-5 RESEARCH ARTICLE Open Access Can early surgery improve the outcome of patients with meconium peritonitis? A single-center experience over 16 years Yi Jiang†, Weihua Pan†, Wenjie Wu, Weipeng Wang, Suna Sun and Jun Wang* Abstract Background: In the last century, meconium peritonitis(MP)was once a highly fatal gastrointestinal. disease With the development of fetal radiological technology, abnormal signs, such as pseudocysts, can. be detected during the fetal period so that more patients can be diagnosed prenatally and receive surgery. in the early stage of life. The survival rate of MP has increased up to 80% in recent years. According to. a review of the treatment and outcomes of patients diagnosed with MP, we evaluated the influence of. early operation on survival rate and discussed the risk factors of prognosis. Methods: We collected 79 cases of patients diagnosed with MP who were treated in our department. from October 2001 to December 2017. They were divided into 2 groups. Patients in group A were born. in our hospital. Patients in group B were born in a local hospital with suspicion of MP and then transferred. to our department. Results: The birth weight (BW) and gestational age (GA) of patients were higher in group A than in. group B. There was no significant difference in the proportion of premature and low birth weight (LBW). patients between the two groups (p=0.422, p=0.970). Their age at the time of surgery was younger in. -

Isolated Fetal Ascites: a Rare Cause
Open Access Case Report DOI: 10.7759/cureus.8433 Isolated Fetal Ascites: A Rare Cause Manikandasamy Veluchamy 1 , Karvendhan Ramasamy 1 , Nishath Liyakat 2 1. Neonatology, NMC Specialty Hospital, Dubai, ARE 2. Neonatology, Zulekha Hospital, Dubai, ARE Corresponding author: Manikandasamy Veluchamy, [email protected] Abstract A moderately preterm, 2.68 kg, male child was born to para 3 live 3 mother by Cesarean delivery done in view of preterm labor with fetal ascites. The baby had antenatally detected ascites. The baby had distended but soft abdomen. Ultrasound abdomen showed gross ascites. X-ray of the abdomen in supine showed faint lucency in the mid-abdomen region posterior to the bowel gas, which was visualized as free gas along the right half of the abdomen in lateral decubitus position, suggestive of bowel perforation. Laparotomy was done on day three of life, intraoperatively found to have perforated Meckel’s diverticulum. Ascites resolved postoperatively. Isolated fetal ascites is a rare condition but has a favorable prognosis. Categories: Pediatrics, Miscellaneous Keywords: isolated fetal ascites, nonimmune hydrops, meconium peritonitis, perforated meckel's diverticulum Introduction Fetal ascites is an abnormal fluid collection in the fetal peritoneal cavity, and it is often the first finding in hydrops fetalis. Ascites is present in 85% of cases of nonimmune hydrops fetalis [1]. Isolated ascites is defined as fluid accumulation in the abdominal cavity without the involvement of fluid accumulation in other body cavities or subcutaneous tissue. It is a rare condition but can be diagnosed easily by ultrasound scanning [2]. Some 30%-75% of cases of isolated fetal ascites resolve spontaneously [3]. -

Newborn Handbook
Pediatric Residency Newborn Handbook 2020-2021 1 Table of Contents Topic Page Contact Information 3 Routine Newborn Care 4 Discharge Talk Guidelines 5-7 AAP Recommendations for Healthy Term Newborn Discharge Criteria 8-9 Basic management of maternal labs/risk factors and Medication Refusal 10-11 Routine Vitamin K Prophylaxis 12 Hep B Vaccine Information and Management of Maternal Hepatitis B Status 13 Routine Erythromycin Prophylaxis for Ophthalmia Neonatorum 14 Hearing Screen 15 CCHD Screening 16 Michigan Newborn Screening 17 Breast Feeding 18-19 Infant feeding policy (donor breast milk) 20 Ankyloglossia and Frenotomy 21 Circumcision 22 Car Seat Safety 23-24 Nursery Protocols 25 Locating Policies, Procedures & Protocols 25 NRP (Neonatal Resuscitation Protocol), APGAR Scoring, MR. SOPA 26 Indirect Hyperbilirubinemia 27-30 Hypoglycemia Algorithm 31-32 Hypoglycemia Treatment, SGA & LGA cutoffs, and Pounds to Kilogram Conversion 33 Chorioamnionitis protocol and antibiotic duration, GBS Algorithm 34-35 Temperature Regulation 36-38 On-Call Problems & a note about SBARs 39 Respiratory/Cardiovascular Respiratory Distress 40-41 Cyanosis 42 Heart Murmurs, Cardiac Exam, and CHD 43 FEN/GI/Endo: Newborn Fluid Management and Weight Specific Guidelines for Feeding 44 Bilious Vomiting 45-46 When You’re Asked About the Appearance of Baby Poop 47 Bloody Stool 48 No stool in 48 hours of life and No void in 30 hours of life 49 Maternal Graves’ Disease 50 Renal Management of Antenatal Hydronephrosis 51 HEENT/Neuro Skull Sutures & Fontanels / Extracerebral Fluid Collections/Subgaleal Hemorrhage 52-53 Infant Fall 54 Oral-facial clefts 55-56 Neonatal Seizures 57 Neonatal Abstinence Syndrome 58-60 Infectious Disease Rubella, CMV, HIV 61 Syphillis 62-63 Toxoplasmosis, HSV 64 Recommended HSV management 64-67 Hepatitis C, Varicella 67 Assessing Gestational Age and the Ballard Score 68 Selected Lab Evaluation 69 Transferring to NICU 70 2 Contact Information Resident ASCOM: 76087. -

Antenatal Appendicular Perforation K.L
Medical Journal 1001-1003 Postgraduate (1987) 63, Postgrad Med J: first published as 10.1136/pgmj.63.745.1001 on 1 November 1987. Downloaded from Antenatal appendicular perforation K.L. Narasimharao, S.K. Mitra and I.C. Pathak Department ofPaediatric Surgery, Postgraduate Institute ofMedical Education and Research, Chandigarh 160 012, India Summary: Antenatal appendicular perforation leading to localized meconium peritonitis and intestinal obstruction is reported in a premature neonate. The baby was successfully treated by a limited ileocaecal resection. Introduction Appendicitis is rare in the neonatal period.'-4 We limited ileocaecal resection was performed with ileo- report a successfully treated case of localized mecon- ascending colon anastomosis (Figure 2). ium peritonitis secondary to an antenatal appen- Histological sections from the terminal ileum and dicular perforation in a premature low birth weight caecum showed normal mucosa and ganglion cells. This is believed to be the second case of Sections from the appendix showed ulceration of the baby. reported Protected by copyright. meconium peritonitis due to in utero appendicular mucosa with increased lymphomononuclear cells in perforation. Case report A 12 day old female neonate, born at 32 weeks gestation, to a third gravida mother by normal vaginal delivery was admitted with constipation and abdominal distension since birth. The antenatal his- tory was unremarkable except for prematurity. She had passed a small amount of meconium at 8 hours after birth. Later, she was able to pass only a few hard and dry pellets occasionally with rectal suppositories. The baby weighed 1400g and the abdomen was http://pmj.bmj.com/ uniformly distended with visible intestinal peristalsis.